
Concept explainers
(a)
Interpretation:
The structural formula for
Concept introduction:
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
Answer to Problem 1E
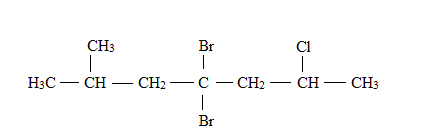
Explanation of Solution
There is a difference between structural formula and condensed formula.
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
In condensed structural formula, the organic structures of the compound are written in a line of text showing all the atoms in the molecule and omitting the vertical and horizontal bonds.
The structural formula for
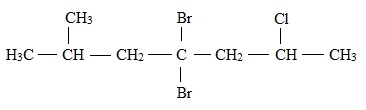
(b)
Interpretation:
The structural formula for
Concept introduction:
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
Answer to Problem 1E
Explanation of Solution
There is a difference between structural formula and condensed formula.
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
In condensed structural formula, the organic structures of the compound are written in a line of text showing all the atoms in the molecule and omitting the vertical and horizontal bonds.
The structural formula for
(c)
Interpretation:
The structural formula for
Concept introduction:
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
Answer to Problem 1E
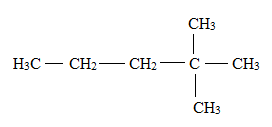
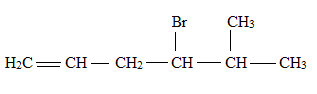
Explanation of Solution
There is a difference between the structural formula and the condensed formula.
The formula which represents the bonding and the type of bonds that hold the atoms in a molecule together is said to be a structural formula.
In condensed structural formula, the organic structures of the compound are written in a line of text showing all the atoms in the molecule and omitting the vertical and horizontal bonds.
The structural formula for
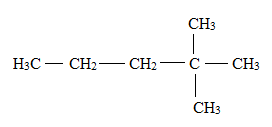
(d)
Interpretation:
The structural formula for
Concept introduction:
The formula which represents the bonding and the type of bonds that hold the atoms in a molecule together is said to be a structural formula.
Answer to Problem 1E
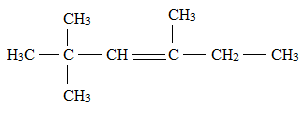
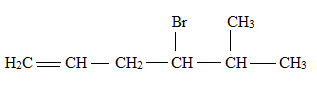
Explanation of Solution
There is a difference between a structural formula and a condensed formula.
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
In condensed structural formula, the organic structures of the compound are written in a line of text showing all the atoms in the molecule and omitting the vertical and horizontal bonds.
The structural formula for
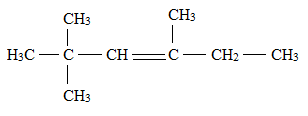
Want to see more full solutions like this?
Chapter 26 Solutions
GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
- 3) Draw a detailed mechanism and predict the product of the reaction shown? 1) EtMgBr 2) H3O+arrow_forwardHow to draw the mechanism for this reaction?arrow_forward> H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





