
(a)
Interpretation:
Styrene can auto initiate free radical
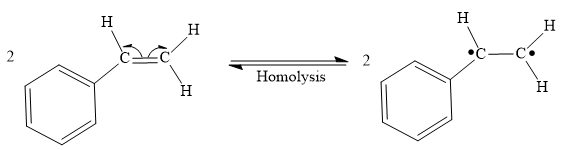
Step 1: Homolysis of the vinyl π bond in a styrene molecule
Step 2: Homolysis of the vinyl π bond in a second styrene molecule
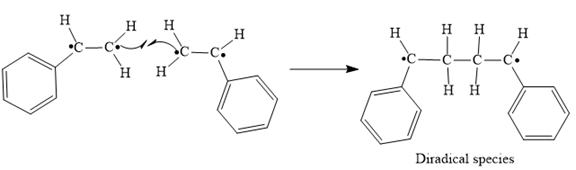
Step 3: Tail-to-tail addition of the radicals from Steps 1 and 2
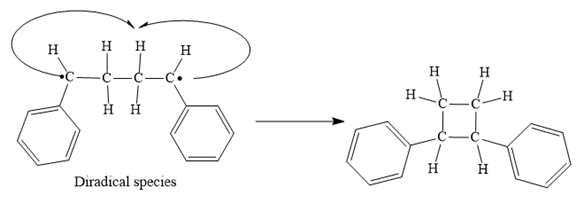
Step 4: Radical coupling of the diradical formed in Step 3.
It as is to be used curved arrow notation to show the steps in a given mechanism.
Concept introduction:
“The breaking of a covalent bond, whereby the electrons making up that bond are distributed equally to the atoms which are disconnected is known as the homolytic bond dissociation or homolysis.” So in homolysis generally radicals are formed. In homolysis, a covalent bond is a breakdown equally and each atom acquires a single electron which is called a radical and a single barbed arrow (![]() ) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest
) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest
Answer to Problem 26.74P
The mechanism for the given steps with curved arrow notation is given below:
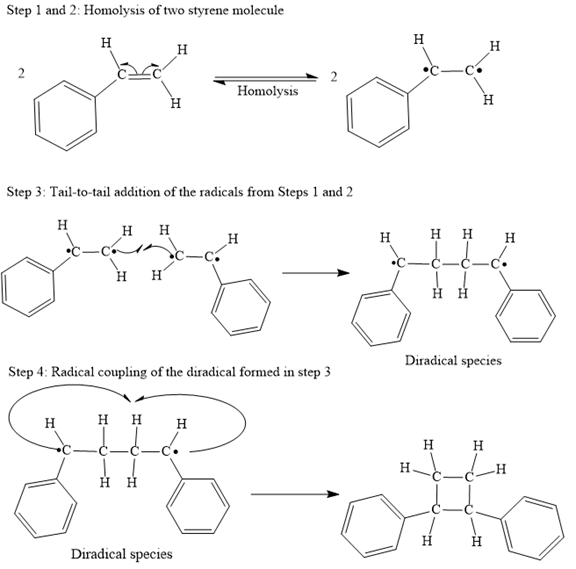
Explanation of Solution
Both steps step 1 and step 2 are same, the homolysis of the vinyl π bond in two styrene molecules.
In step 3 Tail-to-tail addition of the radicals from Steps 1 and 2 takes place as below:
Finally, the radical coupling of the diradical formed in step 3 takes place as below:
A curved arrow notation to show the steps in a given mechanism is used.
(b)
Interpretation:
Styrene can auto initiate free radical polymerization and form polystyrene in the absence of an initiator. At temperatures
Step 1: Homolysis of the vinyl π bond in a styrene molecule
Step 2: Homolysis of the vinyl π bond in a second styrene molecule
Step 3: Tail-to-tail addition of the radicals from Steps 1 and 2
Step 4: Radical coupling of the diradical formed in Step 3.
It is to be explained above steps consistent with those for free radical polymerization.
Concept introduction:
“The breaking of a covalent bond, whereby the electrons making up that bond are distributed equally to the atoms which are disconnected is known as the homolytic bond dissociation or homolysis.” So in homolysis generally radicals are formed. In homolysis, a covalent bond is a breakdown equally and each atom acquires a single electron which is called a radical and a single barbed arrow (![]() ) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest bond dissociation energy. These radials are propagated in the polymerization to elongate the chain of the polymer called a propagation step.
) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest bond dissociation energy. These radials are propagated in the polymerization to elongate the chain of the polymer called a propagation step.
Answer to Problem 26.74P
No, this mechanism is not consistent with a free-radical polymerization, because this mechanism not produces a polymer.
Explanation of Solution
No, this mechanism is not consistent with a free-radical polymerization. In polymerization, the propagation step must occur to elongate the chain of the polymer. But in the given mechanism, the propagation step does not occur thus it not forms polymer, actually, it is a dimer.
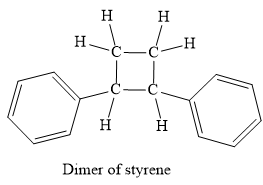
It is explained above steps consistent with those for free radical polymerisation.
(c)
Interpretation:
It is to be explained why the use of an initiator such as benzoyl peroxide more efficient in the synthesis of polystyrene than auto initiated polymerization.
Concept introduction:
“The breaking of a covalent bond, whereby the electrons making up that bond are distributed equally to the atoms which are disconnected is known as the homolytic bond dissociation or homolysis.” So in homolysis generally radicals are formed. In homolysis, a covalent bond is a breakdown equally and each atom acquires a single electron which is called a radical and a single barbed arrow (![]() ) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest bond dissociation energy. These radials are propagated in polymerization are elongated to grow the chain of the polymer called a propagation step.
) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest bond dissociation energy. These radials are propagated in polymerization are elongated to grow the chain of the polymer called a propagation step.
Answer to Problem 26.74P
In case of autoinitiator reaction, the reaction can be terminated too rapidly, because the diradicals formed from the two styrene molecule undergoes dimerization to form the 1, 2-phenylcylcobutane as a product, instead of polymerization. Due to the formation of this product, the growth of a long chain of the molecule does not occur. But with benzoyl peroxide, the radical from the initiation step is a monoradical and it will continue the propagation step to build up polymer, through the chain growth.
Explanation of Solution
In case of autoinitiator reaction, the reaction can be terminated too rapidly, because the diradicals formed from the two styrene molecule undergoes dimerization to form the 1, 2-phenylcylcobutane as a product, instead of polymerization. Due to the formation of this product, the growth of a long chain of the molecule does not occur.

But with benzoyl peroxide, the radical from the initiation step is a monoradical and it will continue the propagation step to build up polymer, through the chain growth.
It is explained why the use of an initiator such as benzoyl peroxide more efficient in the synthesis of polystyrene than auto initiated polymerization.
Want to see more full solutions like this?
Chapter 26 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Indicate the products of the reaction of 2-(3-aminopropyl)cyclohexan-1-one with H2SO4. Draw the structures of the compounds.arrow_forwardIndicate the products of the reaction of 2-cyclopentyl-2-methyl-1,3-dioxolane with H3O+. Draw the structures of the compounds.arrow_forwardQuestion 4 For the molecule shown below, (7 marks): A) Sketch the Newman projection for the view looking along the bond from the perspective of the arrow. B) Then, draw the Newman projection for each 60° rotation along the bond until it returns to the starting point. C) Clearly indicate which Newman projection is the one we see in the structure shown below, and clearly indicate which Newman projection is the highest in energy and which is the lowest in energy. H H Me 'H Me Mearrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts and the amine side product. 'N' 1. NaOH, heat 2. Neutralizing work-up Select to Drawarrow_forwardSubmit Problem 3 of 10 Draw the major product of this reaction. Ignore inorganic byproducts and the amine side product. O 'N' NH 1. NaOH, heat 2. Neutralizing work-up Select to Drawarrow_forwardb) Certain cyclic compounds are known to be conformationally similar to carbohydrates, although they are not themselves carbohydrates. One example is Compound C shown below, which could be imagined as adopting four possible conformations. In reality, however, only one of these is particularly stable. Circle the conformation you expect to be the most stable, and provide an explanation to justify your choice. For your explanation to be both convincing and correct, it must contain not only words, but also "cartoon" orbital drawings contrasting the four structures. Compound C Possible conformations (circle one): Детarrow_forward
- Lab Data The distance entered is out of the expected range. Check your calculations and conversion factors. Verify your distance. Will the gas cloud be closer to the cotton ball with HCI or NH3? Did you report your data to the correct number of significant figures? - X Experimental Set-up HCI-NH3 NH3-HCI Longer Tube Time elapsed (min) 5 (exact) 5 (exact) Distance between cotton balls (cm) 24.30 24.40 Distance to cloud (cm) 9.70 14.16 Distance traveled by HCI (cm) 9.70 9.80 Distance traveled by NH3 (cm) 14.60 14.50 Diffusion rate of HCI (cm/hr) 116 118 Diffusion rate of NH3 (cm/hr) 175.2 175.2 How to measure distance and calculate ratearrow_forwardFor the titration of a divalent metal ion (M2+) with EDTA, the stoichiometry of the reaction is typically: 1:1 (one mole of EDTA per mole of metal ion) 2:1 (two moles of EDTA per mole of metal ion) 1:2 (one mole of EDTA per two moles of metal ion) None of the abovearrow_forwardPlease help me solve this reaction.arrow_forward
- Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





