
(a)
Interpretation:
A mechanism for the initiation step for the
Concept introduction:
The
The polymerizations can be initiated by anions or cations, resulting in anionic polymerization or cationic polymerization, respectively. The polymerization involves, 1) initiation, 2) propagation, and 3) termination.
Initiation is the first step of the polymerization process. An active center is created during initiation, from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators.
In propogation a reactive intermediate is repetitively redeveloped throughout the sequence of a chemical chain reaction. Then it is terminated by adding mono
Answer to Problem 26.42P
The initiation step for the polymerization reaction of the given compound is as follows.
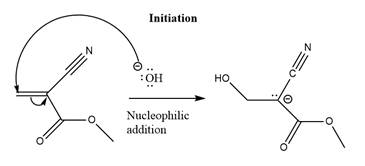
Explanation of Solution
The
The given explanation is obtained by applying the concept polymerization mechanism.
(b)
Interpretation:
A mechanism for the propagation step for the polymerization reaction of the given compound is to be determined.
Concept introduction:
The chemical reaction in which the monomer molecules reacts together to form polymer chains or three dimentional networks is termed as polymerization. This are classified by different syatem avaible for the different types if polymerization.
The polymerizations can be initiated by anions or cations, resulting in anionic polymerization or cationic polymerization, respectively. The polymerization involves, 1) initiation, 2) propagation, and 3) termination.
Initiation is the first step of the polymerization process. An active center is created during initiation, from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators.
In propogation a reactive intermediate is repetitively redeveloped throughout the sequence of a chemical chain reaction. Then it is terminated by adding mono functional groups that having equal of different types of monomer.
Answer to Problem 26.42P
The propagation step for the polymerization reaction of the given compound is as follows.
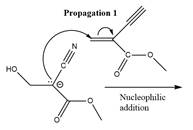
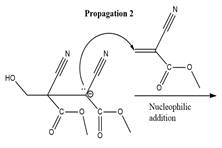
Explanation of Solution
The carbanion formed in initiation step reacts with another equivalent of
in the first nucleophilic addition step. The propagation step repeats the nucleophilic addition reaction.
The given explanation is obtained by applying the concept polymerization mechanism.
(c)
Interpretation:
The structure of
Concept introduction:
The chemical reaction in which the monomer molecules reacts together to form polymer chains or three dimentional networks is termed as polymerization. This are classified by different syatem avaible for the different types if polymerization.
The polymerizations can be initiated by anions or cations, resulting in anionic polymerization or cationic polymerization, respectively. The polymerization involves, 1) initiation, 2) propagation, and 3) termination.
Initiation is the first step of the polymerization process. An active center is created during initiation, from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators.
In propogation a reactive intermediate is repetitively redeveloped throughout the sequence of a chemical chain reaction. Then it is terminated by adding mono functional groups that having equal of different types of monomer.
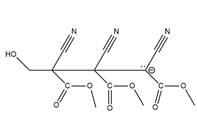
Answer to Problem 26.42P
The structure of
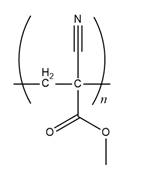
Explanation of Solution
The propagation step repeats the nucleophilic addition reaction to produce
The given explanation is obtained by applying the concept polymerization mechanism.
(d)
Interpretation:
Why is
Concept introduction:
The chemical reaction in which the monomer molecules reacts together to form polymer chains or three dimentional networks is termed as polymerization. This are classified by different syatem avaible for the different types if polymerization.
The polymerizations can be initiated by anions or cations, resulting in anionic polymerization or cationic polymerization, respectively. The polymerization involves, 1) initiation, 2) propagation, and 3) termination.
Initiation is the first step of the polymerization process. An active center is created during initiation, from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators.
In propogation a reactive intermediate is repetitively redeveloped throughout the sequence of a chemical chain reaction. Then it is terminated by adding mono functional groups that having equal of different types of monomer.
Answer to Problem 26.42P
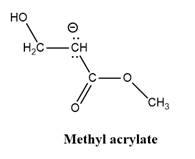
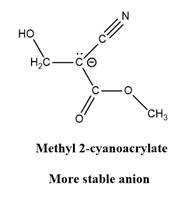
Explanation of Solution
The given explanation is obtained by applying the concept polymerization mechanism, resonance and inductive effect.
(e)
Interpretation:
Why or why not methyl 2-cyanoacrylate would be a good candidate for cationic polymerization is to be explained.
Concept introduction:
The chemical reaction in which the monomer molecules reacts together to form polymer chains or three dimentional networks is termed as polymerization. This are classified by different syatem avaible for the different types if polymerization.
The polymerizations can be initiated by anions or cations, resulting in anionic polymerization or cationic polymerization, respectively. The polymerization involves, 1) initiation, 2) propagation, and 3) termination.
Initiation is the first step of the polymerization process. An active center is created during initiation, from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators.
In propogation a reactive intermediate is repetitively redeveloped throughout the sequence of a chemical chain reaction. Then it is terminated by adding mono functional groups that having equal of different types of monomer.
Answer to Problem 26.42P
Explanation of Solution
The given explanation is obtained by applying the concept polymerization mechanism.
Want to see more full solutions like this?
Chapter 26 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- 8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forward
- о но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forward
- If I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forwardPredict the major organic product(s) for the following reactions.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





