
Concept explainers
Devise a synthesis of each compound from benzene. You may also use any organic compounds having four carbons or fewer, and any required inorganic reagents.
a.  b.
b.  c.
c.  d.
d. 
(a)
Interpretation: A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is to be stated.
Concept introduction: Cyclopropane can be formed by Smith reaction. The reaction of dihalocarbene with the alkene results in addition of carbene to the alkene and this addition takes place from either side of the plane.
Answer to Problem 26.43P
A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is,
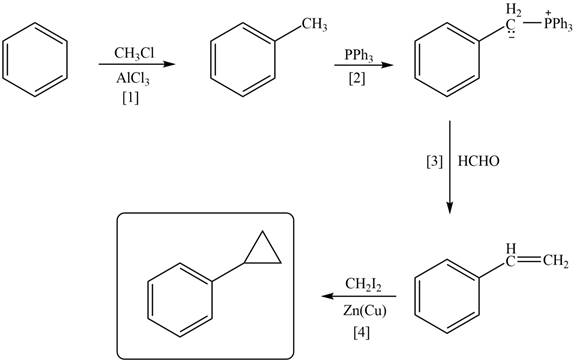
Explanation of Solution
The synthesis of given compound involves three steps.
The first step is the Friedal craft alkylation reaction of benzene with
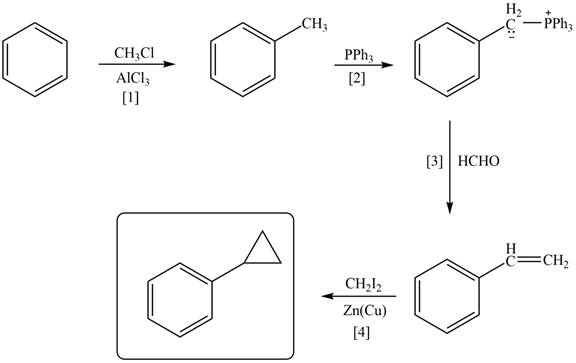
Figure 1
A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is shown in Figure 1.
(b)
Interpretation: A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is to be stated.
Concept introduction: The treatment of
Answer to Problem 26.43P
A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is,
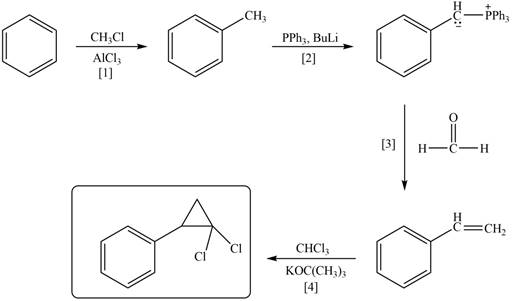
Explanation of Solution
The synthesis of given compound involves four steps.
The first step is Friedel-Craft alkylation of benzene with
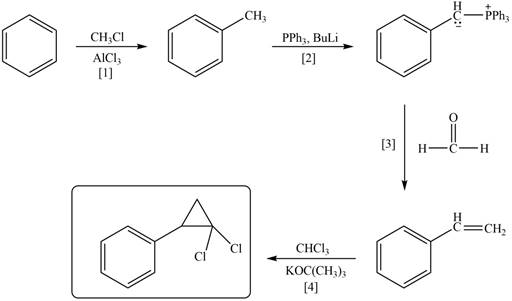
Figure 2
A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is shown in Figure 2.
(c)
Interpretation: A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is to be stated.
Concept introduction: The treatment of an organic halide with an alkene in the presence of
Answer to Problem 26.43P
A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is,
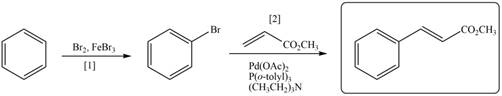
Explanation of Solution
The synthesis of given compound involves two steps.
The first step is bromination of benzene with

Figure 3
A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is shown in Figure 3.
(d)
Interpretation: A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is to be stated.
Concept introduction: The treatment of an organic halide with an alkene in the presence of
Answer to Problem 26.43P
A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is,
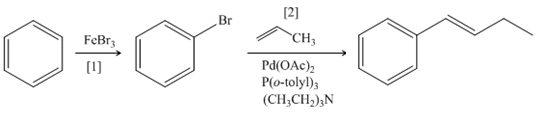
Explanation of Solution
The synthesis of given compound involves two steps.
The first step is bromination of benzene with
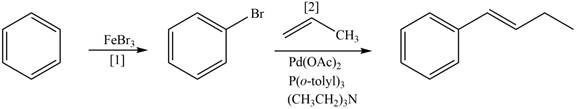
Figure 4
A synthesis of given compound from benzene, any organic compounds having four carbons or fewer, and any required inorganic reagents is shown in Figure 4.
Want to see more full solutions like this?
Chapter 26 Solutions
Organic Chemistry
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





