
Organic Chemistry, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134074580
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 25.8, Problem 20P
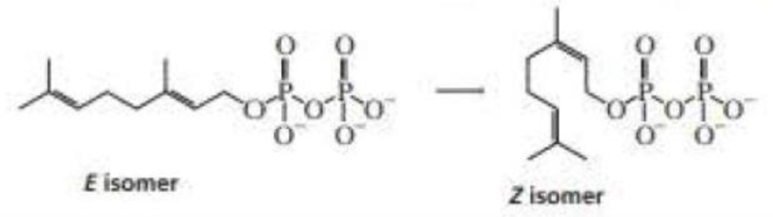
Propose a mechanism for the conversion of the E isomer of geranyl pyrophosphate to the Z isomer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
N
IZ
Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under
the table.
Molecule 1
Molecule 2
HN
Molecule 3
Х
HN
www.
Molecule 4
Molecule 5
Molecule 6
none of the above
NH
NH
G
Show work with explanation. don't give Ai generated solution
Follow the curved arrows to draw a second resonance structure for each species. Explain and steps for individual understanding.
Chapter 25 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
Ch. 25.1 - Prob. 1PCh. 25.3 - Which has a higher melting point, glyceryl...Ch. 25.3 - Draw the structure of an optically inactive fat...Ch. 25.3 - Draw the structure of an optically active fat...Ch. 25.5 - Do the identities of R1 and R2 in phosphatidic...Ch. 25.5 - Membranes contain proteins, Integral membrane...Ch. 25.5 - Prob. 8PCh. 25.5 - The membrane phospholipids in deer have a higher...Ch. 25.6 - Treating PGC2 with a strong base such as sodium...Ch. 25.7 - Mark off the isoprene units in menthol, -selinene,...
Ch. 25.7 - Prob. 13PCh. 25.7 - Prob. 14PCh. 25.8 - Propose mechanisms for the Claisen condensation...Ch. 25.8 - Prob. 16PCh. 25.8 - Propose a mechanism for the conversion of...Ch. 25.8 - Propose a mechanism for the biosynthesis of...Ch. 25.8 - Propose a mechanism for the conversion of the E...Ch. 25.8 - The fluoro-substitued geranyl pyrophosphate shown...Ch. 25.8 - Prob. 22PCh. 25.8 - Prob. 23PCh. 25.9 - Draw the individual 1,2-hydride and 1,2-methyl...Ch. 25.10 - Prob. 26PCh. 25.10 - Prob. 27PCh. 25.10 - The acid component of a cholesterol ester is a...Ch. 25.10 - Prob. 29PCh. 25.10 - Prob. 30PCh. 25 - Prob. 31PCh. 25 - An optically active fat, when completely...Ch. 25 - Prob. 33PCh. 25 - a. How many different triacylglycerols are there...Ch. 25 - Cardiolipins are found in heart muscles. Draw the...Ch. 25 - Nutmeg contains a simple, fully saturated...Ch. 25 - Draw the product that is obtained from the...Ch. 25 - Prob. 39PCh. 25 - Prob. 40PCh. 25 - Propose a mechanism for the biosynthesis of...Ch. 25 - 5-Androstene-3.17-dione is isomerized to...Ch. 25 - Prob. 44PCh. 25 - Eudesmol is a sesquiterpene found in eucalyptus....Ch. 25 - Prob. 46PCh. 25 - Prob. 47PCh. 25 - Diethylstilbestrol (DES) was given to pregnant...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw all reasonable resonance structures for the following cation. Then draw the resonance hybrid. Provide steps and explanationarrow_forwardHow are the molecules or ions in each pair related? Classify them as resonance structures, isomers, or neither.arrow_forwardWhich of the given resonance structures (A, B, or C) contributes most to the resonance hybrid? Which contributes least? Provide steps and explanationarrow_forward
- Substance X is known to exist at 1 atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of these other properties of X have been determined: melting point enthalpy of fusion 90. °C 8.00 kJ/mol boiling point 130. °C enthalpy of vaporization 44.00 kJ/mol density 2.80 g/cm³ (solid) 36. J.K mol (solid) 2.50 g/mL (liquid) heat capacity 32. J.Kmol (liquid) 48. J.Kmol (vapor) You may also assume X behaves as an ideal gas in the vapor phase. Ex Suppose a small sample of X at 50 °C is put into an evacuated flask and heated at a constant rate until 15.0 kJ/mol of heat has been added to the sample. Graph the temperature of the sample that would be observed during this experiment. o0o 150- 140 130- 120- 110- 100- G Ar ?arrow_forwardMechanism. Provide the mechanism for the reaction below. You must include all arrows, intermediates, and formal charges. If drawing a Sigma complex, draw all major resonance forms. The ChemDraw template of this document is available on Carmen. Br FeBr3 Brarrow_forwardCheck the box under each compound that exists as a pair of mirror-image twins. If none of them do, check the none of the above box under the table. CH3 OH CH3 CH2 -CH-CH3 CH3 OH OH CH-CH2-CH- -CH3 CH3 CH3 OH OH CH3 C -CH2- C. -CH3 CH3- -CH2- -CH-CH2-OH OH CH3 none of the above كarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you


 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT



Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License