
Organic Chemistry, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134074580
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 25, Problem 43P
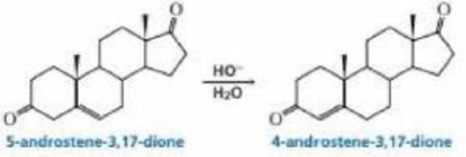
5-Androstene-3.17-dione is isomerized to 4-androstene-3.17-dione by hydroxide ion propose a mechanism for this reaction
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Construct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)
please help with hw
help me solve this hw
Chapter 25 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
Ch. 25.1 - Prob. 1PCh. 25.3 - Which has a higher melting point, glyceryl...Ch. 25.3 - Draw the structure of an optically inactive fat...Ch. 25.3 - Draw the structure of an optically active fat...Ch. 25.5 - Do the identities of R1 and R2 in phosphatidic...Ch. 25.5 - Membranes contain proteins, Integral membrane...Ch. 25.5 - Prob. 8PCh. 25.5 - The membrane phospholipids in deer have a higher...Ch. 25.6 - Treating PGC2 with a strong base such as sodium...Ch. 25.7 - Mark off the isoprene units in menthol, -selinene,...
Ch. 25.7 - Prob. 13PCh. 25.7 - Prob. 14PCh. 25.8 - Propose mechanisms for the Claisen condensation...Ch. 25.8 - Prob. 16PCh. 25.8 - Propose a mechanism for the conversion of...Ch. 25.8 - Propose a mechanism for the biosynthesis of...Ch. 25.8 - Propose a mechanism for the conversion of the E...Ch. 25.8 - The fluoro-substitued geranyl pyrophosphate shown...Ch. 25.8 - Prob. 22PCh. 25.8 - Prob. 23PCh. 25.9 - Draw the individual 1,2-hydride and 1,2-methyl...Ch. 25.10 - Prob. 26PCh. 25.10 - Prob. 27PCh. 25.10 - The acid component of a cholesterol ester is a...Ch. 25.10 - Prob. 29PCh. 25.10 - Prob. 30PCh. 25 - Prob. 31PCh. 25 - An optically active fat, when completely...Ch. 25 - Prob. 33PCh. 25 - a. How many different triacylglycerols are there...Ch. 25 - Cardiolipins are found in heart muscles. Draw the...Ch. 25 - Nutmeg contains a simple, fully saturated...Ch. 25 - Draw the product that is obtained from the...Ch. 25 - Prob. 39PCh. 25 - Prob. 40PCh. 25 - Propose a mechanism for the biosynthesis of...Ch. 25 - 5-Androstene-3.17-dione is isomerized to...Ch. 25 - Prob. 44PCh. 25 - Eudesmol is a sesquiterpene found in eucalyptus....Ch. 25 - Prob. 46PCh. 25 - Prob. 47PCh. 25 - Diethylstilbestrol (DES) was given to pregnant...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Briefly explain chemical potential.arrow_forwardReason whether it is possible to determine changes in the Galvani potential difference at the metal-solution interface.arrow_forwardObtain the standard potential at 25°C of the Cu* I Cu | Pt electrode from the standard potentials E° Cu²+/Cu = 0.341 V and E Cu²+ /Cu+ = 0.153 V.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY