
College Physics
1st Edition
ISBN: 9781938168000
Author: Paul Peter Urone, Roger Hinrichs
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 25, Problem 8PE
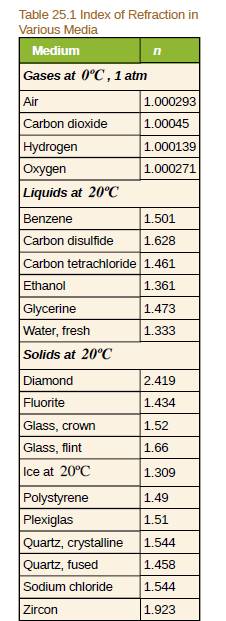
In what substance in Table 25.1 is the
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Can someone help me answer this physics 2 questions. Thank you.
Four capacitors are connected as shown in the figure below. (Let C = 12.0 μF.)
a
C
3.00 με
Hh.
6.00 με
20.0 με
HE
(a) Find the equivalent capacitance between points a and b.
5.92
HF
(b) Calculate the charge on each capacitor, taking AV ab = 16.0 V.
20.0 uF capacitor 94.7
6.00 uF capacitor 67.6
32.14
3.00 µF capacitor
capacitor C
☑
με
με
The 3 µF and 12.0 uF capacitors are in series and that combination is in parallel with the 6 μF capacitor. What quantity is the same for capacitors in parallel? μC
32.14
☑
You are correct that the charge on this capacitor will be the same as the charge on the 3 μF capacitor. μC
In the pivot assignment, we observed waves moving on a string stretched by hanging
weights. We noticed that certain frequencies produced standing waves. One such
situation is shown below:
0 ст
Direct Measurement
©2015 Peter Bohacek I.
20
0 cm 10
20
30
40
50
60
70
80
90
100
Which Harmonic is this?
Do NOT include units!
What is the wavelength of this wave in cm with only no decimal places?
If the speed of this wave is 2500 cm/s, what is the frequency of this harmonic (in Hz, with
NO decimal places)?
Chapter 25 Solutions
College Physics
Ch. 25 - Using the law of reflection, explain how powder...Ch. 25 - Diffusion by reflection from a rough surface is...Ch. 25 - Why is the index of refraction always greater than...Ch. 25 - Does the fact that the light flash from lightning...Ch. 25 - Will light change direction toward or away from...Ch. 25 - Explain why an object in water always appears to...Ch. 25 - Explain why a person’s legs appeal very short when...Ch. 25 - Why is the front surface of a thermometer curved...Ch. 25 - Suppose light were incident from air onto a...Ch. 25 - A ring with a colorless gemstone is dropped into...
Ch. 25 - A high-quality diamond may be quite clear and...Ch. 25 - Is it possible that total internal reflection...Ch. 25 - The most common type at mirage is an illusion that...Ch. 25 - It can he argued that a flat piece of glass, such...Ch. 25 - You can often see a reflection when looking at a...Ch. 25 - When you focus a camera, you adjust the distance...Ch. 25 - A thin lens has two focal points, one on either...Ch. 25 - Will the focal length of a lens change when it is...Ch. 25 - What are the differences between teal and virtual...Ch. 25 - Can you see a virtual image? Can you photograph...Ch. 25 - Is it necessary to project a real image onto a...Ch. 25 - At what distance is an image always locatedat do,...Ch. 25 - Under what circumstances will an image be located...Ch. 25 - What is meant by a negative magnification? What is...Ch. 25 - Can a case 1 image be larger than the object even...Ch. 25 - Figure 25.49 shows a light bulb between two...Ch. 25 - Devise an arrangement of mirrors allowing you to...Ch. 25 - If you wish to see your entire body in a flat...Ch. 25 - It can be argued than a flat mirror has an in?nite...Ch. 25 - Why are diverging mirrors often used for rear-view...Ch. 25 - Suppose a man stands in front of a mirror as shown...Ch. 25 - Show that when light reflects from two mirrors...Ch. 25 - Light shows staged with lasers use moving mirrors...Ch. 25 - A flat minor is neither converging nor diverging....Ch. 25 - What is the speed of light in water? In glycerine?Ch. 25 - What is the speed of light in air? In crown glass?Ch. 25 - Calculate the index of refraction for a medium in...Ch. 25 - In what substance in Table 25.1 is the speed of...Ch. 25 - There was a major collision of an asteroid with...Ch. 25 - A scuba diver training in a pool looks at his...Ch. 25 - Components of some computers communicate with each...Ch. 25 - (a) Using information in Figure 25.53, find the...Ch. 25 - Suppose you have an unknown clear substance...Ch. 25 - On the Moon’s surface, lunar astronauts placed a...Ch. 25 - Suppose Figure 25.54 represents a ray of light...Ch. 25 - Figure 25.54 shows a ray of light passing from one...Ch. 25 - Unreasonable Results Suppose light travels from...Ch. 25 - Construct Your Own Problem Consider sunlight...Ch. 25 - Unreasonable Results Light traveling from water to...Ch. 25 - Verify that the critical angle for light going...Ch. 25 - (a) At the end of Example 25.4, it was stated that...Ch. 25 - An optical fiber uses flint glass clad with crown...Ch. 25 - At what minimum angle will you get total internal...Ch. 25 - Suppose you are using total internal reflection to...Ch. 25 - You can determine me index of refraction of a...Ch. 25 - A ray of light, emitted beneath the surface of an...Ch. 25 - A light ray entering an optical fiber surrounded...Ch. 25 - (a) What is me ratio of the speed of red light to...Ch. 25 - A beam of white light goes from air into water at...Ch. 25 - By how much do the critical angles for red (660...Ch. 25 - (a) A narrow beam of light containing yellow (580...Ch. 25 - A parallel beam of light containing orange (610...Ch. 25 - A ray of 610 nm light goes from air into fused...Ch. 25 - A narrow beam of light containing red (660 nm) and...Ch. 25 - A narrow beam of white light enters a prism made...Ch. 25 - What is the power in diopters at a camera lens...Ch. 25 - Your camera's zoom lens has an adjustable focal...Ch. 25 - What is the focal length of 1.75 D reading glasses...Ch. 25 - You note that your prescription for new eyeglasses...Ch. 25 - How far from the lens must the film in a camera...Ch. 25 - A certain slide projector has a 100 mm focal...Ch. 25 - A doctor examines a mole with a 15.0 cm focal...Ch. 25 - How far from a piece of paper must you hold your...Ch. 25 - A camera with a 50.0 mm focal length lens is being...Ch. 25 - A camera lens used for taking close-up photographs...Ch. 25 - Suppose your 50.00 mm local length camera lens is...Ch. 25 - (a) What is the focal length of a magnifying glass...Ch. 25 - What magnification will be produced by a lens of...Ch. 25 - In Example 25.7, the magnification of a book held...Ch. 25 - Suppose a 200 mm focal length telephoto lens is...Ch. 25 - A camera with a 100 mm focal length lens is used...Ch. 25 - Combine thin lens equations to show that the...Ch. 25 - What is the focal length of a makeup mirror that...Ch. 25 - Some telephoto cameras use a mirror rather than a...Ch. 25 - (a) Calculate the focal length of the mirror...Ch. 25 - Find the magnification of the heater element in...Ch. 25 - What is the focal length of a makeup mirror that...Ch. 25 - A shopper standing 3.00 m from a convex security...Ch. 25 - An object 1.50 cm high is held 3.00 cm from a...Ch. 25 - Ray tracing for a flat mirror shows that the image...Ch. 25 - Show that for a flat mirror hi= ho, knowing that...Ch. 25 - Use the law of reflection to prove that the focal...Ch. 25 - Referring to the electric room heater considered...Ch. 25 - Consider a 250-W heat lamp fixed to the ceiling in...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Explain why 92% of 2,4-pemtanedione exists as the enol tautomer in hexane but only 15% of this compound exists ...
Organic Chemistry (8th Edition)
1. A person gets in an elevator on the ground floor and rides it to the top floor of a building. Sketch a veloc...
College Physics: A Strategic Approach (3rd Edition)
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties (2nd Edition)
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
Endospore formation is called (a) _____. It is initiated by (b) _____. Formation of a new cell from an endospor...
Microbiology: An Introduction
Some organizations are starting to envision a sustainable societyone in which each generation inherits sufficie...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Four capacitors are connected as shown in the figure below. (Let C = 12.0 µF.) A circuit consists of four capacitors. It begins at point a before the wire splits in two directions. On the upper split, there is a capacitor C followed by a 3.00 µF capacitor. On the lower split, there is a 6.00 µF capacitor. The two splits reconnect and are followed by a 20.0 µF capacitor, which is then followed by point b. (a) Find the equivalent capacitance between points a and b. µF(b) Calculate the charge on each capacitor, taking ΔVab = 16.0 V. 20.0 µF capacitor µC 6.00 µF capacitor µC 3.00 µF capacitor µC capacitor C µCarrow_forwardTwo conductors having net charges of +14.0 µC and -14.0 µC have a potential difference of 14.0 V between them. (a) Determine the capacitance of the system. F (b) What is the potential difference between the two conductors if the charges on each are increased to +196.0 µC and -196.0 µC? Varrow_forwardPlease see the attached image and answer the set of questions with proof.arrow_forward
- How, Please type the whole transcript correctly using comma and periods as needed. I have uploaded the picture of a video on YouTube. Thanks,arrow_forwardA spectra is a graph that has amplitude on the Y-axis and frequency on the X-axis. A harmonic spectra simply draws a vertical line at each frequency that a harmonic would be produced. The height of the line indicates the amplitude at which that harmonic would be produced. If the Fo of a sound is 125 Hz, please sketch a spectra (amplitude on the Y axis, frequency on the X axis) of the harmonic series up to the 4th harmonic. Include actual values on Y and X axis.arrow_forwardSketch a sign wave depicting 3 seconds of wave activity for a 5 Hz tone.arrow_forward
- Sketch a sine wave depicting 3 seconds of wave activity for a 5 Hz tone.arrow_forwardThe drawing shows two long, straight wires that are suspended from the ceiling. The mass per unit length of each wire is 0.050 kg/m. Each of the four strings suspending the wires has a length of 1.2 m. When the wires carry identical currents in opposite directions, the angle between the strings holding the two wires is 20°. (a) Draw the free-body diagram showing the forces that act on the right wire with respect to the x axis. Account for each of the strings separately. (b) What is the current in each wire? 1.2 m 20° I -20° 1.2 marrow_forwardplease solve thisarrow_forward
- please solve everything in detailarrow_forward6). What is the magnitude of the potential difference across the 20-02 resistor? 10 Ω 11 V - -Imm 20 Ω 10 Ω 5.00 10 Ω a. 3.2 V b. 7.8 V C. 11 V d. 5.0 V e. 8.6 Varrow_forward2). How much energy is stored in the 50-μF capacitor when Va - V₁ = 22V? 25 µF b 25 µF 50 µFarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Polarization of Light: circularly polarized, linearly polarized, unpolarized light.; Author: Physics Videos by Eugene Khutoryansky;https://www.youtube.com/watch?v=8YkfEft4p-w;License: Standard YouTube License, CC-BY