
Concept explainers
Interpretation:
Using octadecanoic (stearic) acid and any necessary organic and inorganic reagents, an efficient synthesis for each compound is to be described.
Concept introduction:
The reduction of
The primary alcohol on oxidation with pyridinium dichromate
In Clemmensen reduction, the carbonyl group (aldehyde or
The esters can be synthesized by acid catalyzed condensation of carboxylic acid with alcohol.
The ester on reaction with one molar equivalent of Grignard’s reagent in diethyl ether gives ketone by carbon-carbon bond formation.
The ester on reaction with two molar equivalents of Grignard’s reagent in diethyl ether gives tertiary alcohol.
The dehydration of alcohol is the loss of
The alcohol on acid catalyzed dehydration gives corresponding alkene.
The alkene on hydrogenation with the catalyst undergoes addition of hydrogen across the double bond and forms an alkane.
The primary amine can be prepared by the acylation of ammonia.
The secondary amide can be prepared by the nucleophilic substitution of acyl chloride by amine. The two moles amines used with one mole of acyl chloride, because one amine molecule acts as a nucleophile and second acts as a Brønsted base.
The carboxylic acids on reaction with thionyl chloride forms acyl chloride by replacing the hydroxyl group of carboxylic acid with chlorine atom.
The primary amide on reduction with lithium aluminum hydride
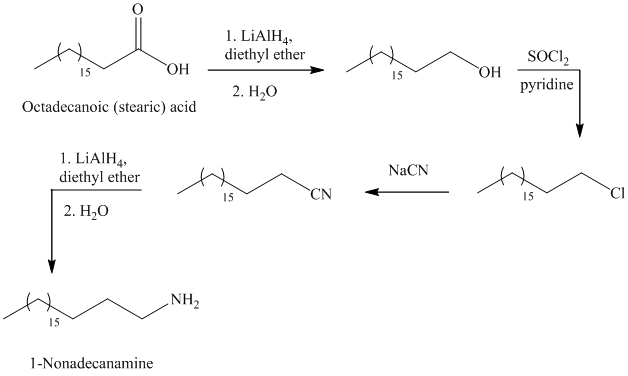
The reaction of thionyl chloride with alcohol gives alkyl halide.
The reaction of alkyl halide with sodium cyanide gives alkyl cyanide.
The cyanide (nitrile) can be reduced to primary amine using lithium aluminum hydride
The alkyl bromide can be prepared by the reaction of alcohol with phosphorus tribromide
Grignard reagents are prepared by the reaction of the magnesium metal with an alkyl or aryl halide usually in diethyl ether as the solvent.
The potassium or sodium dichromate in presence of strong acid forms chromic acid which is a good oxidizing agent, in hydrous medium oxidizes primary alcohol to carboxylic acid.
The epoxide on treatment with Grignard reagent undergoes epoxide ring opening by forming a corresponding alcohol.
Answer to Problem 28P
Solution:
a)
b)
c)
d) 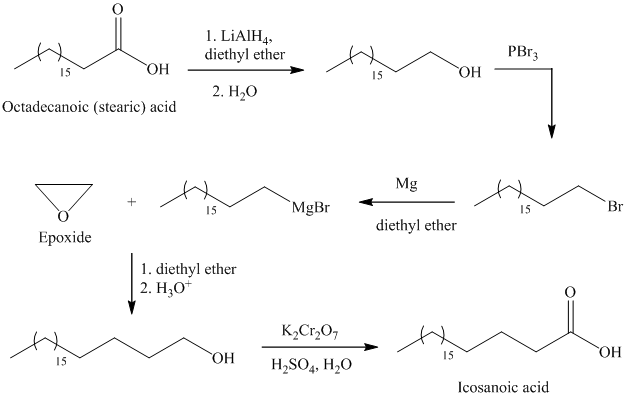
e) 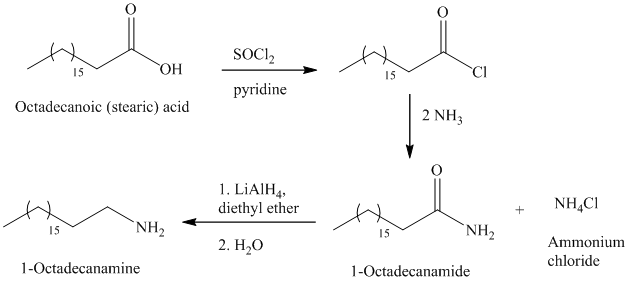
f)
Explanation of Solution
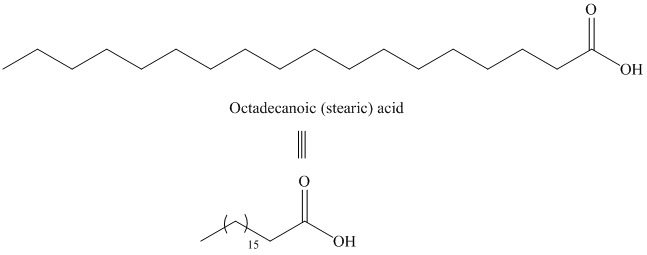
The structure of octadecanoic (stearic) acid is shown below:
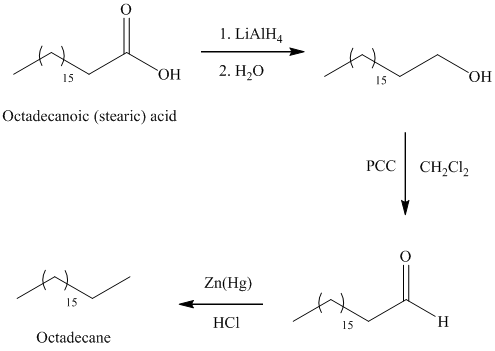
a) Octadecane
The synthesis of octadecane from octadecanoic acid can be done by following reactions sequence:
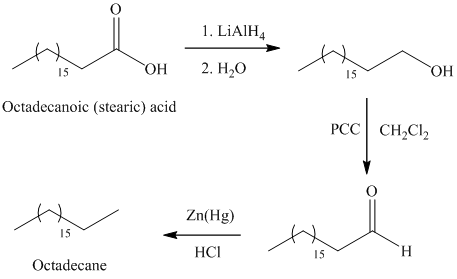
The octadecanoic acid on reaction with lithium aluminum hydride in aqueous medium reduced to octadecanol which is further on oxidation with pyridinium dichromate
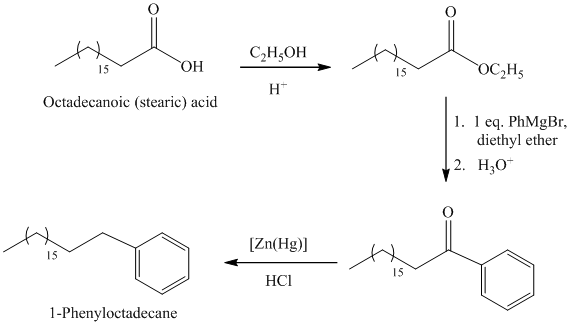
b)
The synthesis of
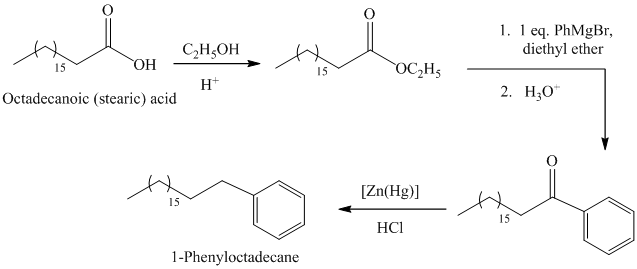
The octadecanoic acid first converted to an ester by reacting it with ethanol in acidic condition. The ester formed is then reacted with a Grignard’s reagent phenylmagnesium bromide
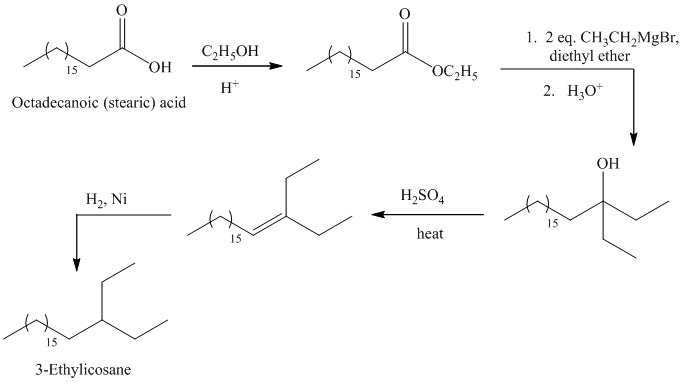
c)
The synthesis of
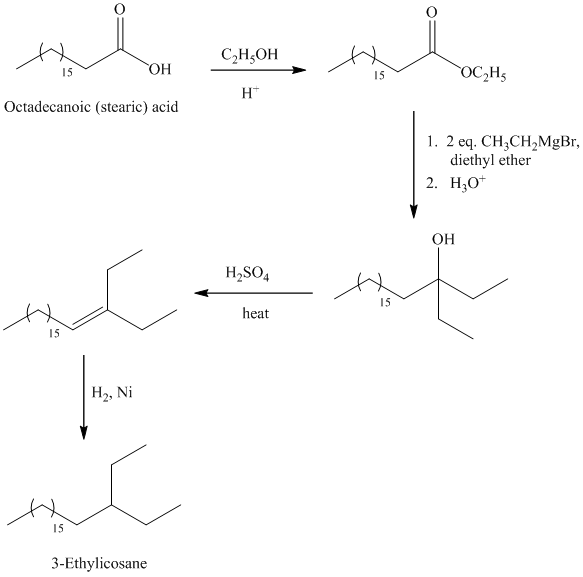
The octadecanoic acid is first converted to an ester by reacting it with ethanol in acidic condition. The ester formed is then reacted with a Grignard’s reagent ethyl bromide bromide
d) Icosanoic acid
The synthesis of Icosanoic acid from octadecanoic acid can be done by following reactions sequence:
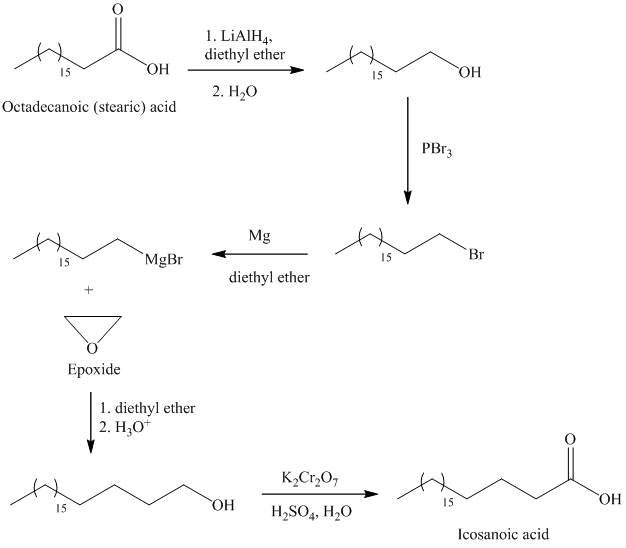
In the first step, the octadecanoic acid is reduced to primary alcohol by reducing agent lithium aluminum hydride
e)
The synthesis of
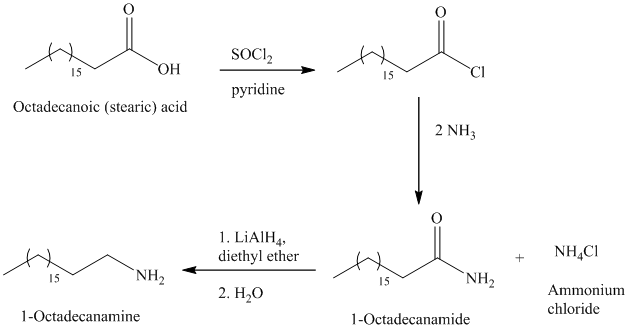
The octadecanoic acid on reaction with thonyl chloride in presence of pyridine gave the product of acyl chloride. The acyl chloride is converted to
f)
The synthesis of
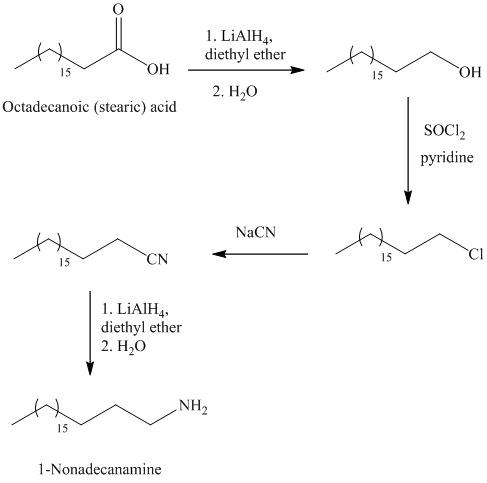
In the first step, the octadecanoic acid is reduced to primary alcohol by reducing agent lithium aluminum hydride
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry - Standalone book
- Calculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forwardThe following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forward
- The following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forwardIf we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forwardBriefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forward
- Explain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 101.7 mL of a 0.3500M solution of piperidine (C5H10NH) with a 0.05700M solution of HClO4. The pK of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 682.9 mL of the HClO solution to it. 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO solution added. 4 Round your answer to 2 decimal places. pH = .11 00. 18 Ararrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0 262.7 QUESTION: For both groups of data provide answers to the calculations attached in the imagearrow_forward
- 7. Concentration and uncertainty in the estimate of concentration (class data) Class mean for sample (Regular) |[Cl-] (mmol/L) class mean Sn za/2 95% Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Confidence Interval (mg/100 mL)arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardGive reason(s) for six from the followings [using equations if possible] a. Addition of sodium carbonate to sulfanilic acid in the Methyl Orange preparation. b. What happened if the diazotization reaction gets warmed up by mistake. c. Addition of sodium nitrite in acidified solution in MO preparation through the diazotization d. Using sodium dithionite dihydrate in the second step for Luminol preparation. e. In nitroaniline preparation, addition of the acid mixture (nitric acid and sulfuric acid) to the product of step I. f. What is the main reason of the acylation step in nitroaniline preparation g. Heating under reflux. h. Fusion of an organic compound with sodium. HAND WRITTEN PLEASEarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





