
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
8th Edition
ISBN: 9781305582439
Author: Brown, William H.; Iverson, Brent L.; Anslyn, Eric; Foote, Christopher S.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 24.5, Problem 24.6P
Interpretation Introduction
Interpretation:
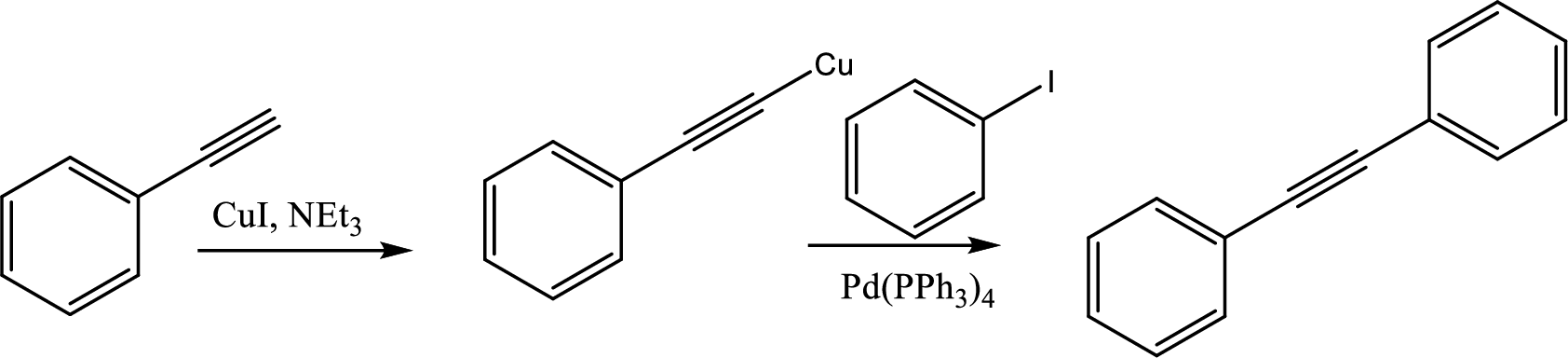
The sequence of the given reaction has to be given.
Concept Introduction:
Sonogashira coupling starts with a terminal alkyne with
This coupling is used to create diaryl
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Highlight the chirality (or stereogenic) center(s) in the given compound. A compound may have one or more stereogenic centers.
OH
OH
OH
OH
OH
OH
Using wedge-and-dash bonds, modify the bonds on the chiral carbon in the molecule below so the molecule has R stereochemical configuration.
NH
H
Br
X
टे
Provide photos of models of the following molecules. (Include a key for identification of the atoms)
1,2-dichloropropane
2,3,3-trimethylhexane
2-bromo-3-methybutane
Chapter 24 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
Ch. 24.3 - Prob. 24.1PCh. 24.3 - Prob. 24.2PCh. 24.4 - Prob. 24.3PCh. 24.5 - Show how the following compound can be prepared...Ch. 24.5 - Prob. 24.5PCh. 24.5 - Prob. 24.6PCh. 24.6 - Prob. 24.7PCh. 24 - Prob. 24.8PCh. 24 - Prob. 24.9PCh. 24 - Prob. 24.10P
Ch. 24 - Treatment of cyclohexene with iodobenzene under...Ch. 24 - Prob. 24.12PCh. 24 - Prob. 24.13PCh. 24 - The aryl diene undergoes sequential Heck reactions...Ch. 24 - Heck reactions take place with alkynes as well as...Ch. 24 - Prob. 24.16PCh. 24 - The following transformation involves a series of...Ch. 24 - Show the sequence of Heck reactions by which the...Ch. 24 - Prob. 24.19PCh. 24 - Write the steps that are critical in the following...Ch. 24 - Prob. 24.21PCh. 24 - Prob. 24.22PCh. 24 - Prob. 24.23PCh. 24 - Show how the following compound could be prepared...Ch. 24 - It is typically very difficult to do a...Ch. 24 - The compound eutypine is an antibacterial agent...Ch. 24 - Prob. 24.27PCh. 24 - Prob. 24.28PCh. 24 - Prob. 24.29PCh. 24 - Prob. 24.30PCh. 24 - Prob. 24.31PCh. 24 - Prob. 24.32PCh. 24 - Prob. 24.33PCh. 24 - The following transformation can be accomplished...Ch. 24 - Prob. 24.35PCh. 24 - Prob. 24.36PCh. 24 - Prob. 24.37PCh. 24 - Prob. 24.38PCh. 24 - E. J. Coreys 1964 total synthesis of...Ch. 24 - Prob. 24.40P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFirefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.arrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardGiven a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forward
- TRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forwardRelative Transmittance 0.995 0.99 0.985 0.98 Please draw the structure that is consistent with all the spectral data below in the box and alphabetically label the equivalent protons in the structure (Ha, Hb, Hc ....) in order to assign all the proton NMR peaks. Label the absorption bands in the IR spectrum indicated by the arrows. INFRARED SPECTRUM 1 0.975 3000 2000 Wavenumber (cm-1) 1000 Structure with assigned H peaks 1 3 180 160 140 120 100 f1 (ppm) 80 60 40 20 0 C-13 NMR note that there are 4 peaks between 120-140ppm Integral values equal the number of equivalent protons 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 fl (ppm)arrow_forwardCalculate the pH of 0.0025 M phenol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License