
(a)
Interpretation:
The types of
Concept introduction:
Functional groups:
The functional group is defined as an atom or group of atoms combined in a specific manner that gives the chemical properties of the organic compound and they are the main reason for the chemical reactivity of the compound. Compounds having similar functional groups undergoes similar type of reactions.
(b)
Interpretation:
The number of chiral centres present in estrone that has to be calculated.
Concept introduction:
Chiral centre:
Chiral centre is defined as an atom bonded to four different chemical species. It is a stereo centre that holds the atom in such way that the structure may not be superimposable to its mirror image. They give optical isomerism.
(c)
Interpretation:
Structural formula for the compounds
Concept introduction:
Structural formula:
The structural formula of a compound can be defined as a graphic representation of the molecular structure showing how the atoms are arranged and the
Retrosynthetic approach:
Retrosynthetic analysis is a technique for planning a synthesis mainly for complex organic molecules where the complex target molecule is reduced into a sequence of progressively simpler structures along a pathway which ultimately leads to the identification of a simple starting molecule or easily available from which the synthesis can be developed.
During retrosynthetic analysis the target molecule is symmetrically broken down by a combination of functional group interconversion and disconnection. This disconnection is related to breaking of carbon-carbon bond of a molecule to generate simpler fragments. The complete set of disconnections and functional group interconversions for a specified target molecule is what constitutes a retrosynthetic plan.
Heck reaction:
The Heck reaction is the
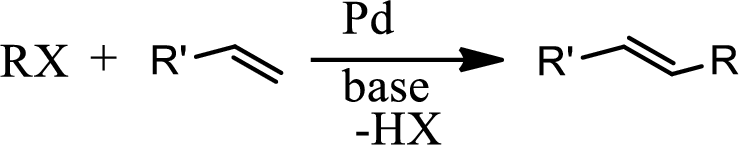
(d)
Interpretation:
The pathway of converting compounds
Concept introduction:
Heck reaction:
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in presence of a base and palladium catalyst to form a substituted alkene. The reaction is as follows,
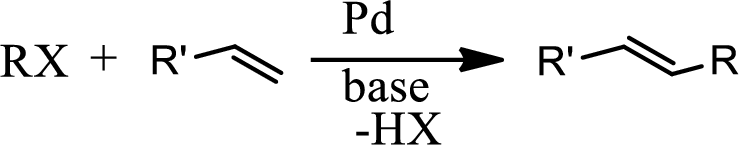
(e)
Interpretation:
The stereochemistry of compound
Concept introduction:
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in presence of a base and palladium catalyst to form a substituted alkene. The reaction is as follows,
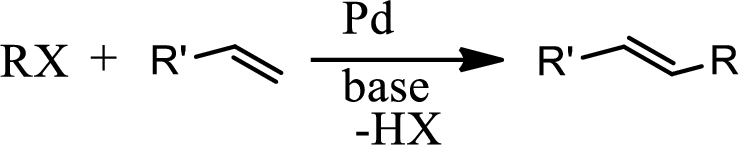
(f)
Interpretation:
The pathway of conversion of tertiary butyl ether to acetone to form estrone from compound
Concept introduction:
Oxidation of secondary alcohol:
Oxidation of alcohol to carbonyl is very difficult as the reaction is so fast that it always ends up by giving acid. Hence for secondary alcohol to get oxidised to carbonyl selectively PCC is used.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Given a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forwardTRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forward
- Relative Transmittance 0.995 0.99 0.985 0.98 Please draw the structure that is consistent with all the spectral data below in the box and alphabetically label the equivalent protons in the structure (Ha, Hb, Hc ....) in order to assign all the proton NMR peaks. Label the absorption bands in the IR spectrum indicated by the arrows. INFRARED SPECTRUM 1 0.975 3000 2000 Wavenumber (cm-1) 1000 Structure with assigned H peaks 1 3 180 160 140 120 100 f1 (ppm) 80 60 40 20 0 C-13 NMR note that there are 4 peaks between 120-140ppm Integral values equal the number of equivalent protons 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 fl (ppm)arrow_forwardCalculate the pH of 0.0025 M phenol.arrow_forwardIn the following reaction, the OH- acts as which of these? NO2-(aq) + H2O(l) ⇌ OH-(aq) + HNO2(aq)arrow_forward
- Using spectra attached, can the unknown be predicted? Draw the predicition. Please explain and provide steps. Molecular focrmula:C16H13ClOarrow_forwardCalculate the percent ionization for 0.0025 M phenol. Use the assumption to find [H3O+] first. K = 1.0 x 10-10arrow_forwardThe Ka for sodium dihydrogen phosphate is 6.32 x 10-8. Find the pH of a buffer made from 0.15 M H2PO4- and 0.25 M HPO42- .arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

