
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24.5, Problem 12P
What product is formed when each
a.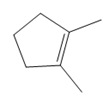 b.
b. 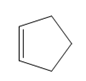 c.
c. 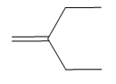
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Find the pH of a 0.120 M solution of HNO2.
Find the pH ignoring activity effects (i.e., the normal way).
Find the pH in a solution of 0.050 M NaCl, including activity
Please help me answer these three questions. Required info should be in data table.
Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given
nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each
stereogenic center. Omit any byproducts.
Bri
CH3CH2O-
(conc.)
Draw the major organic product or products.
Chapter 24 Solutions
Organic Chemistry (6th Edition)
Ch. 24.1 - Prob. 1PCh. 24.1 - Prob. 2PCh. 24.1 - Prob. 3PCh. 24.2 - Prob. 4PCh. 24.2 - Prob. 5PCh. 24.4 - Problem 26.10
What reagents are needed to convert...Ch. 24.5 - Problem 26.11
What product is formed when each...Ch. 24.5 - Prob. 13PCh. 24.6 - Problem 26.13
Draw the products formed when each...Ch. 24.6 - Problem 26.14
What products are formed when ...
Ch. 24 - 26.19 What product is formed by ring-closing...Ch. 24 - 26.20 Draw the products formed in each...Ch. 24 - What organic halide is needed to convert lithium...Ch. 24 - 26.22 How can you convert ethynylcyclohexane to...Ch. 24 - 26.23 What compound is needed to convert styrene...Ch. 24 - 26.24 What steps are needed to convert to octane...Ch. 24 - Prob. 27PCh. 24 - 26.27 Draw the products (including stereoisomers)...Ch. 24 - 26.28 Treatment of cyclohexene with and forms...Ch. 24 - Prob. 32PCh. 24 - 26.30 What starting material is needed to prepare...Ch. 24 - Prob. 37PCh. 24 - Prob. 38PCh. 24 - When certain cycloalkenes are used in metathesis...Ch. 24 - 26.34 Draw the products formed in each reaction.
...Ch. 24 - Prob. 41PCh. 24 - Draw a stepwise mechanism for the following...Ch. 24 - Sulfur ylides, like the phosphorus ylides of...Ch. 24 - Although diazomethane is often not a useful...Ch. 24 - Prob. 46PCh. 24 - Prob. 47PCh. 24 - 26.45 Devise a synthesis of each compound from...Ch. 24 - 26.46 Devise a synthesis of each substituted...Ch. 24 - Biaryls, compounds containing two aromatic rings...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Tartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forward
- Including activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forwardCan I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forward
- Ordene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forwardState the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forward
- Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction. For the decomposition reaction of N2O5(g): 2 N2O5(g) · 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 -> NO2 + NO3_(K1) NO2 + NO3 →> N2O5 (k-1) → NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Give the expression for the acceptable rate. (A). d[N₂O] dt = -1 2k,k₂[N205] k₁+k₂ d[N₂O5] (B). dt =-k₁[N₂O₂] + k₁[NO2][NO3] - k₂[NO2]³ (C). d[N₂O] dt =-k₁[N₂O] + k₁[N205] - K3 [NO] [N205] (D). d[N2O5] =-k₁[NO] - K3[NO] [N₂05] dtarrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 20.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY