
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24, Problem 40P
Draw the products formed in each reaction.
a. 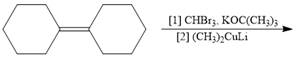 e.
e. 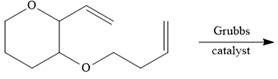
b. 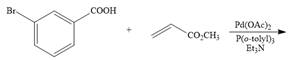 f.
f. 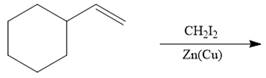
c. 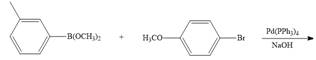 g.
g. 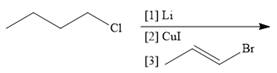
d. 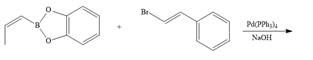 h.
h. 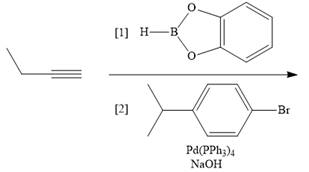
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Choose the Lewis structure for the compound below:
H2CCHOCH2CH(CH3)2
HH
H
:d
H
H
H C.
Η
H
H
HH
H
H
H
H.
H
H
H
HH
H
H
H
H
H-
H
H
H
C-H
H
H
HHHH
Each of the highlighted carbon atoms
is connected to
hydrogen atoms.
く
Complete the reaction in the drawing area below by adding the major products to the right-hand side.
If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area
instead.
Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge
bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center.
More...
No reaction.
Explanation
Check
O
+
G
1. Na O Me
Click and drag to start
drawing a structure.
2. H
+
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
000
Ar
P
Chapter 24 Solutions
Organic Chemistry (6th Edition)
Ch. 24.1 - Prob. 1PCh. 24.1 - Prob. 2PCh. 24.1 - Prob. 3PCh. 24.2 - Prob. 4PCh. 24.2 - Prob. 5PCh. 24.4 - Problem 26.10
What reagents are needed to convert...Ch. 24.5 - Problem 26.11
What product is formed when each...Ch. 24.5 - Prob. 13PCh. 24.6 - Problem 26.13
Draw the products formed when each...Ch. 24.6 - Problem 26.14
What products are formed when ...
Ch. 24 - 26.19 What product is formed by ring-closing...Ch. 24 - 26.20 Draw the products formed in each...Ch. 24 - What organic halide is needed to convert lithium...Ch. 24 - 26.22 How can you convert ethynylcyclohexane to...Ch. 24 - 26.23 What compound is needed to convert styrene...Ch. 24 - 26.24 What steps are needed to convert to octane...Ch. 24 - Prob. 27PCh. 24 - 26.27 Draw the products (including stereoisomers)...Ch. 24 - 26.28 Treatment of cyclohexene with and forms...Ch. 24 - Prob. 32PCh. 24 - 26.30 What starting material is needed to prepare...Ch. 24 - Prob. 37PCh. 24 - Prob. 38PCh. 24 - When certain cycloalkenes are used in metathesis...Ch. 24 - 26.34 Draw the products formed in each reaction.
...Ch. 24 - Prob. 41PCh. 24 - Draw a stepwise mechanism for the following...Ch. 24 - Sulfur ylides, like the phosphorus ylides of...Ch. 24 - Although diazomethane is often not a useful...Ch. 24 - Prob. 46PCh. 24 - Prob. 47PCh. 24 - 26.45 Devise a synthesis of each compound from...Ch. 24 - 26.46 Devise a synthesis of each substituted...Ch. 24 - Biaryls, compounds containing two aromatic rings...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
Give the IUPAC name for each compound.
Organic Chemistry
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a tetramer of this alternating copolymer.arrow_forwardH I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forwardPolylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forward
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY