
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
4th Edition
ISBN: 9781260562620
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 24, Problem 98CP
Interpretation Introduction
Interpretation:
The amount of ATP is produced from the complete catabolism of one molecule of triglycerol should be determined.
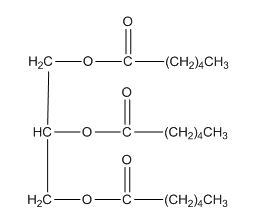
Concept introduction:
ATP (adenosine triphosphate) is the main source of energy for the cells. It is found in all forms of life. For this reason, ATP is often termed as 'energy currency'. During
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Given the first-order reaction: aA → products. State its kinetic equation.
Determine the symmetry of the
combination of atomic orbitals for bf
4-
H
Draw the product and the mechanism
1.) LDA
+
2.) H30*
H
○ excess
OH
Chapter 24 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
Ch. 24.2 - Analyze the following reaction by considering the...Ch. 24.2 - Prob. 24.2PPCh. 24.3 - Prob. 24.1PCh. 24.3 - Prob. 24.2PCh. 24.3 - Prob. 24.3PCh. 24.3 - Prob. 24.4PCh. 24.3 - Prob. 24.5PCh. 24.3 - Prob. 24.6PCh. 24.4 - Prob. 24.7PCh. 24.4 - Prob. 24.8P
Ch. 24.4 - Prob. 24.9PCh. 24.5 - Prob. 24.10PCh. 24.5 - Prob. 24.11PCh. 24.5 - Prob. 24.12PCh. 24.6 - Prob. 24.13PCh. 24.7 - Prob. 24.14PCh. 24.7 - Prob. 24.3PPCh. 24.7 - Prob. 24.15PCh. 24.7 - Prob. 24.16PCh. 24.7 - Use the number of molecules of ATP formed from the...Ch. 24.7 - Prob. 24.18PCh. 24.8 - Prob. 24.19PCh. 24.8 - Prob. 24.20PCh. 24.8 - Prob. 24.21PCh. 24.9 - Prob. 24.4PPCh. 24.9 - What products are formed when each amino acid is...Ch. 24.9 - Prob. 24.22PCh. 24 - Analyze each reaction by considering the...Ch. 24 - Analyze each reaction by considering the...Ch. 24 - Prob. 25PCh. 24 - Prob. 26PCh. 24 - Prob. 27PCh. 24 - Prob. 28PCh. 24 - Prob. 29PCh. 24 - Prob. 30PCh. 24 - Prob. 31PCh. 24 - Prob. 32PCh. 24 - Glucose is completely metabolized to six molecules...Ch. 24 - Why is glycolysis described as an anaerobic...Ch. 24 - Write the overall equation with key coenzymes for...Ch. 24 - Prob. 36PCh. 24 - Prob. 37PCh. 24 - Prob. 38PCh. 24 - Consider the aerobic and anaerobic avenues of...Ch. 24 - Prob. 40PCh. 24 - Prob. 41PCh. 24 - Prob. 42PCh. 24 - Prob. 43PCh. 24 - Prob. 44PCh. 24 - Prob. 45PCh. 24 - Prob. 46PCh. 24 - Prob. 47PCh. 24 - Prob. 48PCh. 24 - Prob. 49PCh. 24 - Prob. 50PCh. 24 - Prob. 51PCh. 24 - Prob. 52PCh. 24 - Prob. 53PCh. 24 - Prob. 54PCh. 24 - Prob. 55PCh. 24 - Prob. 56PCh. 24 - Prob. 57PCh. 24 - Prob. 58PCh. 24 - Prob. 59PCh. 24 - How much ATP is generated by the complete...Ch. 24 - Prob. 61PCh. 24 - Fill in the boxes with the number of moles of each...Ch. 24 - Prob. 63PCh. 24 - Prob. 64PCh. 24 - Prob. 65PCh. 24 - Prob. 66PCh. 24 - Prob. 67PCh. 24 - Prob. 68PCh. 24 - Prob. 69PCh. 24 - Prob. 70PCh. 24 - What is the difference between ketogenic and...Ch. 24 - Prob. 72PCh. 24 - Prob. 73PCh. 24 - Draw the structure of the keto acid formed by the...Ch. 24 - Draw the products formed in each transamination...Ch. 24 - Prob. 76PCh. 24 - Prob. 77PCh. 24 - Prob. 78PCh. 24 - Prob. 79PCh. 24 - Prob. 80PCh. 24 - What metabolic intermediate is formed from the...Ch. 24 - What metabolic intermediate is formed from the...Ch. 24 - Prob. 83PCh. 24 - Prob. 84PCh. 24 - Prob. 85PCh. 24 - Prob. 86PCh. 24 - Prob. 87PCh. 24 - What is the cause of the pain and cramping in a...Ch. 24 - Prob. 89PCh. 24 - Prob. 90PCh. 24 - Prob. 91PCh. 24 - Prob. 92PCh. 24 - Prob. 93PCh. 24 - Prob. 94PCh. 24 - What type of enzyme would catalyze the conversion...Ch. 24 - Prob. 96PCh. 24 - Prob. 97CPCh. 24 - Prob. 98CPCh. 24 - Prob. 99CPCh. 24 - Prob. 100CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
- Given the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forwardThe structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning