
Concept explainers
a)
Interpretation:
A reaction that leads to the formation of triglyceride, starting with glycerol and carboxylic acids has to be suggested.
Concept introduction:
Ester formation reaction: Reaction of alcohol and

a)
Explanation of Solution
The hydroxyl group act as nucleophile and the carboxylic group act as electrophile in presence of acid catalyst; the nucleophile attack at electrophilic carbon of carboxylic acid leads to the formation of ester with the elimination of water molecule.
Mechanism of condensation reaction:
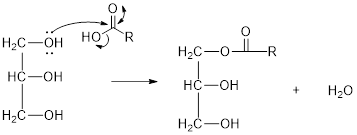
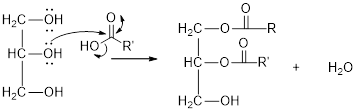
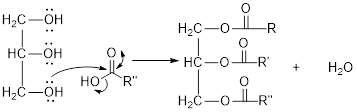
As shown above, the successive steps lead to the formation of triglycerides containing three ester group with the elimination of three water molecules.
b)
Interpretation:
An equation for the base hydrolysis of ester has to be written.
Concept introduction:
Ester formation reaction: Reaction of alcohol and carboxylic acid using acid catalyst results the ester formation with the elimination of water molecule.

b)
Explanation of Solution
The hydroxyl group acts as nucleophile and the carbonyl carbon act as electrophile; the nucleophile attack at electrophilic carbon of ester leads to the formation of alcohol with the elimination of fatty acid salts (soap).
Base hydrolysis of Esters:
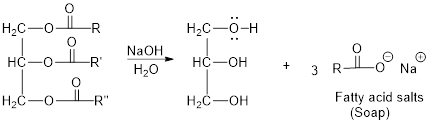
c)
Interpretation:
Difference between fats and oils has to be explained.
Concept introduction:
Melting point: At temperature begins the solid to melt.
Unsaturation bonds: The presence of double or triple bonds in the molecules.
c)
Explanation of Solution
The presence of unsaturated bonds in the molecules tight close packing will be less due to bend of double bonds and the intermolecular attraction between them is less and less energy is required to overcome the interaction. More the double bonds lower the intermolecular interaction. Hence, the melting point decreases.
d)
Interpretation:
Reagent and catalyst used in hydrogenation process has to be identified.
Concept introduction:
Hydrogenation of
Homogeneous catalyst: Catalyst used is in same phase as the reactants.
Heterogeneous catalyst: Catalyst used is in different phase as the reactants.
d)
Explanation of Solution
Liquid oil is obtained from plants, having double bonds the presence of reactive double bond is converted into single bonds in order to solidify. Hydrogenation of double bonds is the process in which hydrogen molecule is added across the double bond forming alkane product. The alkane is highly facilitated for close packing and solidifies the oil.
Reaction carried out is hydrogenation reaction; hydrogen molecule is the reagent used in presence of either heterogeneous or homogeneous catalyst.
e)
Interpretation:
Iodine number has to be calculated.
Concept introduction:
Iodine number: number of grams of Iodine that react with given quantity of oil is called Iodine number.
Number of moles = Molarity
e)
Explanation of Solution
Given: molarity of
Number of moles of
The mol ratio between
Number of grams of
The iodine number is the number of grams of iodine that reacts with 100 g of corn oil.
Hence, Iodine number calculated is 123
Want to see more full solutions like this?
Chapter 24 Solutions
Chemistry
- Please help answer number 2. Thanks in advance.arrow_forwardHow do I explain this? Thank you!arrow_forwardWhen an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/mol (M* = 163 m/z) is formed. In the infrared spectrum, important absorptions appear at 1661, 750 and 690 cm. The 13C NMR and DEPT spectra are provided. Draw the structure of the product as the resonance contributor lacking any formal charges. 13C NMR DEPT 90 200 160 120 80 40 0 200 160 120 80 40 0 DEPT 135 T 200 160 120 80 40 0 Draw the unknown amide. Select Dow Templates More Fragearrow_forward
- Identify the unknown compound from its IR and proton NMR spectra. C4H6O: 'H NMR: 82.43 (1H, t, J = 2 Hz); 8 3.41 (3H, s); 8 4.10 (2H, d, J = 2 Hz) IR: 2125, 3300 cm¹ The C4H6O compound liberates a gas when treated with C2H5 MgBr. Draw the unknown compound. Select Draw с H Templates Morearrow_forwardPlease help with number 6 I got a negative number could that be right?arrow_forward1,4-Dimethyl-1,3-cyclohexadiene can undergo 1,2- or 1,4-addition with hydrogen halides. (a) 1,2-Addition i. Draw the carbocation intermediate(s) formed during the 1,2-addition of hydrobromic acid to 1,4-dimethyl-1,3-cyclohexadiene. ii. What is the major 1,2-addition product formed during the reaction in (i)? (b) 1,4-Addition i. Draw the carbocation intermediate(s) formed during the 1,4-addition of hydrobromic acid to 1,4-dimethyl-1,3-cyclohexadiene. ii. What is the major 1,4-addition product formed from the reaction in (i)? (c) What is the kinetic product from the reaction of one mole of hydrobromic acid with 1,4-dimethyl-1,3-cyclohexadiene? Explain your reasoning. (d) What is the thermodynamic product from the reaction of one mole of hydrobro-mic acid with 1,4-dimethyl-1,3-cyclohexadiene? Explain your reasoning. (e) What major product will result when 1,4-dimethyl-1,3-cyclohexadiene is treated with one mole of hydrobromic acid at - 78 deg * C ? Explain your reasoning.arrow_forward
- Give the product of the bimolecular elimination from each of the isomeric halogenated compounds. Reaction A Reaction B. КОВ CH₂ HotBu +B+ ко HOIBU +Br+ Templates More QQQ Select Cv Templates More Cras QQQ One of these compounds undergoes elimination 50x faster than the other. Which one and why? Reaction A because the conformation needed for elimination places the phenyl groups and to each other Reaction A because the conformation needed for elimination places the phenyl groups gauche to each other. ◇ Reaction B because the conformation needed for elimination places the phenyl groups gach to each other. Reaction B because the conformation needed for elimination places the phenyl groups anti to each other.arrow_forwardFive isomeric alkenes. A through each undergo catalytic hydrogenation to give 2-methylpentane The IR spectra of these five alkenes have the key absorptions (in cm Compound Compound A –912. (§), 994 (5), 1643 (%), 3077 (1) Compound B 833 (3), 1667 (W), 3050 (weak shoulder on C-Habsorption) Compound C Compound D) –714 (5), 1665 (w), 3010 (m) 885 (3), 1650 (m), 3086 (m) 967 (5), no aharption 1600 to 1700, 3040 (m) Compound K Match each compound to the data presented. Compound A Compound B Compound C Compound D Compoundarrow_forward7. The three sets of replicate results below were accumulated for the analysis of the same sample. Pool these data to obtain the most efficient estimate of the mean analyte content and the standard deviation. Lead content/ppm: Set 1 Set 2 Set 3 1. 9.76 9.87 9.85 2. 9.42 9.64 9.91 3. 9.53 9.71 9.42 9.81 9.49arrow_forward
- Draw the Zaitsev product famed when 2,3-dimethylpentan-3-of undergoes an El dehydration. CH₂ E1 OH H₁PO₁ Select Draw Templates More QQQ +H₂Oarrow_forwardComplete the clean-pushing mechanism for the given ether synthesia from propanol in concentrated sulfurica140°C by adding any mining aloms, bands, charges, nonbonding electron pairs, and curved arrows. Draw hydrogen bonded to cayan, when applicable. ore 11,0 HPC Step 1: Draw curved arrows Step 2: Complete the intend carved Q2Q 56 QQQ Step 3: Complete the intermediate and add curved Step 4: Modify the structures to draw the QQQ QQQarrow_forward6. In an experiment the following replicate set of volume measurements (cm3) was recorded: (25.35, 25.80, 25.28, 25.50, 25.45, 25.43) A. Calculate the mean of the raw data. B. Using the rejection quotient (Q-test) reject any questionable results. C. Recalculate the mean and compare it with the value obtained in 2(a).arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





