
(a)
Interpretation:
Empirical formula of the given substance has to be predicted.
Concept introduction:
Steps to calculate empirical formula:
- Convert the mass of elements into moles.
- Divide each mole value by the smallest number of moles calculated.
- Round to the nearest whole number.
Number of moles = Molarity
(a)
Explanation of Solution
Calculate moles of each given elements:
This gives the formula
b)
Interpretation:
Does the substance behave as an ideal gas has to be predicted.
Concept introduction:
Ideal gas equation:
Boyle’s law: The pressure of a given mass of an ideal gas is inversely proportional to its volume at a constant temperature.
b)
Explanation of Solution
When temperature and amount of gas are constant, the product of pressure times volume is constant (Boyle’s law).
For given pressure and volume values,
If the number of moles and temperature are remains constant, then the product of pressure and volume should be same. If not then substances does not behave as an ideal gas.
As shown above none of the values are same. Hence, the substances do not behave as an ideal gas.
c)
Interpretation:
The molecular formula has to be predicted.
Concept introduction:
Steps to calculate empirical formula:
- Convert the mass of elements into moles.
- Divide each mole value by the smallest number of moles calculated.
- Round to the nearest whole number.
Number of moles = Molarity
c)
Explanation of Solution
Calculate moles of each given elements:
This gives the formula
Now, let’s calculate moles using the ideal gas equation, and then calculate the molar mass.
The formula mass of
d)
Interpretation:
Lewis structure of the molecule and its geometry has to be drawn and described.
Concept introduction:
Structural Isomerism: Structural Isomers are the structure of a molecule with same molecular formula but have different arrangements of bonds and atoms and position of double bond also changes from more substituted to less substituted or vice-versa.
Lewis structure: The bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Geometric isomers of
Cis-isomer: When two particular atoms (group of atoms) are adjacent to each other, the alkene is known as cis-isomer.
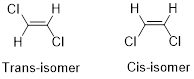
Trans-isomer: When two particular atoms (group of atoms) are across from each other, the alkene is known as trans-isomer.
d)
Explanation of Solution
Compound
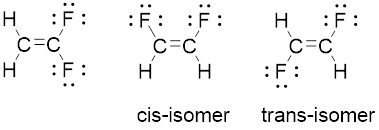
The geometry of each carbon is trigonal planar. Arrangement of two identical fluorine atoms on the same side adjacent to each other known as cis-isomer. And represnted opposite side to each other known as trans-isomer.
e)
Interpretation:
The systematic name of the structure has to be written.
Concept introduction:
- The longest continuous chain of carbon atoms is identified.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
Geometric isomers of Alkenes:
Cis-isomer: When two particular atoms (group of atoms) are adjacent to each other, the alkene is known as cis-isomer.
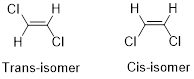
Trans-isomer: When two particular atoms (group of atoms) are across from each other, the alkene is known as trans-isomer.
e)
Explanation of Solution
Given name: cis-2-butene
Predict the longest continuous chain of carbon atoms:
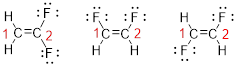
The parent name is ETHENE represent the longest chain of carbon atoms contains two carbons. The Suffix ‘ene’ represents presence of double bond at C-1.
Predict substituents and its location:
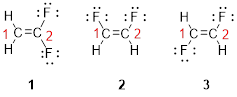
The first compound structure has two fluorine atoms located at carbon-1. Hence the name can be written as substituent followed by parent name; 2,2-difluoroethene.
The second compound structure has two fluorine atoms located at carbon-1and 2. The term ‘cis-’ indicates two fluorine atoms are located adjacent to each other on same side. Hence the name can be written as substituent followed by parent name; cis-1,2-difluoroethene.
The third compound structure has two fluorine atoms located at carbon-1and 2. The term ‘trans-’ indicates two fluorine atoms are located opposite to each other. Hence the name can be written as substituent followed by parent name; trans-1,2-difluoroethene.
Want to see more full solutions like this?
Chapter 24 Solutions
CHEMISTRY (LOOSELEAF) >CUSTOM<
- Understanding the general acid-base properties of amino acids O Proteins Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule The solution is... 010 H3N-CH-C-OH CH HO CH3 O acidic O basic neutral O (unknown) H3N HO 0 O acidic O basic neutral ○ (unknown) H3N-CH-C-O CH2 CH3-CH-CH3 O acidic O basic Oneutral ○ (unknown) O= X H2N-CH-C-O CH3 CH CH3 acidic O basic O neutral ○ (unknown) ? 000arrow_forwardImagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule 0=0 H3N-CH-C-o HO CH2 OH The solution is... O acidic O basic O neutral O (unknown) H₂N acidic O basic O neutral ○ (unknown) + H3N O OH O acidic O basic O neutral O (unknown) H2N-CH-C-O CH3 O acidic O basic neutral ○ (unknown) X ? olo HEarrow_forwardRecognizing ampli Draw an a amino acid with a methyl (-CH3) side chain. Explanation Check Click and drag to start drawing a structure. X Carrow_forward
- Write the systematic name of each organic molecule: structure name × HO OH ☐ OH CI CI O CI OH OHarrow_forwardく Check the box under each a amino acid. If there are no a amino acids at all, check the "none of them" box under the table. Note for advanced students: don't assume every amino acid shown must be found in nature. COO H3N-C-H CH2 HO CH3 NH3 O CH3-CH CH2 OH Onone of them Explanation Check + H3N O 0. O OH + NH3 CH2 CH3-CH H2N C-COOH H O HIC + C=O H3N-C-O CH3- - CH CH2 OH Х 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardWrite the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forward
- theres 2 productsarrow_forwardDraw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forward
- Organic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forwardDifferentiate between electrophilic and nucleophilic groups. Give examples.arrow_forwardAn aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


