
Chemistry
13th Edition
ISBN: 9781259911156
Author: Raymond Chang Dr., Jason Overby Professor
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 24, Problem 24.31QP
a)
Interpretation Introduction
Interpretation:
The structure for 1-bromo-3-methylbenzene has to be drawn.
Concept introduction:
- The parent compound of aromatic compound is Benzene.
- The substituent groups attached to the parent is identified. A substituent group contains group of atoms attached to the carbon atom of the aromatic ring.
- While numbering the parent, the location of the second group relative to the first substituent uses prefixes like o-ortho (1,2-), m-meta (1,3-), and p-para (1,4) in disubstituted aromatic ring.
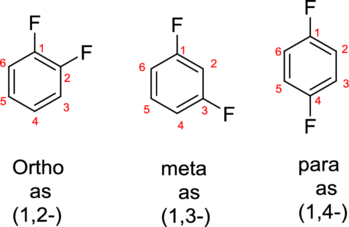
b)
Interpretation Introduction
Interpretation:
The structure for 1-chloro-2-propyl-benzene has to be drawn.
Concept introduction:
Refer part ‘a’
c)
Interpretation Introduction
Interpretation:
The structure for 1,2,4,5-tetramethylbenzene has to be drawn.
Concept introduction:
Refer part ‘a’
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
PLEASE help. Locate a literature IR spectrum of eugenol. Insert the literature spectrum here:
What conclusions can you draw about your clove oil from these IR spectra? I attached my data below
please help and the percent recovery of clove oil from cloves is 4.61% and i have attached my ir spectrum as well.
Based on your GC data, how many components are in the clove oil?
Calculate the percentage of each component. Clearly show your work.
Which of the components corresponds to eugenol? How do you know?
Is eugenol the major component?
please help and i am so confused if the picture is the gc data or ir spectrum. you dont have to do everything just what you can please because i am lost and the mass of the cloves was Mass of cloves 62.299g. Mass of recovered clove oil 62.761g.
Chapter 24 Solutions
Chemistry
Ch. 24.2 - How many structural isomers are there in the...Ch. 24.2 - Prob. 2PECh. 24.2 - Prob. 3PECh. 24.2 - Prob. 4PECh. 24.2 - Prob. 1RCFCh. 24.2 - For which of the following compounds are cis-trans...Ch. 24.3 - Prob. 1RCFCh. 24.4 - Prob. 5PECh. 24.4 - Prob. 1RCFCh. 24 - Prob. 24.1QP
Ch. 24 - Prob. 24.2QPCh. 24 - What do saturated and unsaturated mean when...Ch. 24 - Prob. 24.4QPCh. 24 - Prob. 24.5QPCh. 24 - Why is it that alkanes and alkynes, unlike...Ch. 24 - Prob. 24.7QPCh. 24 - Prob. 24.8QPCh. 24 - Prob. 24.9QPCh. 24 - Give examples of a chiral substituted alkane and...Ch. 24 - Draw all possible structural isomers for the...Ch. 24 - Prob. 24.12QPCh. 24 - Draw all possible isomers for the molecule C4H8.Ch. 24 - Draw all possible isomers for the molecule C3H5Br.Ch. 24 - Prob. 24.15QPCh. 24 - Prob. 24.16QPCh. 24 - Draw the structures of cis-2-butene and...Ch. 24 - Prob. 24.18QPCh. 24 - Prob. 24.19QPCh. 24 - Prob. 24.20QPCh. 24 - Prob. 24.21QPCh. 24 - Prob. 24.22QPCh. 24 - Prob. 24.23QPCh. 24 - Prob. 24.24QPCh. 24 - Which of the following amino acids are chiral: (a)...Ch. 24 - Name the following compounds: (a) CH3CCCH2CH3Ch. 24 - Prob. 24.27QPCh. 24 - Prob. 24.28QPCh. 24 - Prob. 24.29QPCh. 24 - Prob. 24.30QPCh. 24 - Prob. 24.31QPCh. 24 - Name the following compounds:Ch. 24 - Prob. 24.33QPCh. 24 - Prob. 24.34QPCh. 24 - Prob. 24.35QPCh. 24 - Prob. 24.36QPCh. 24 - Prob. 24.37QPCh. 24 - Prob. 24.38QPCh. 24 - Prob. 24.39QPCh. 24 - Prob. 24.40QPCh. 24 - Predict the product or products of each of the...Ch. 24 - Prob. 24.42QPCh. 24 - Prob. 24.43QPCh. 24 - Given these data...Ch. 24 - Prob. 24.45QPCh. 24 - Prob. 24.46QPCh. 24 - Prob. 24.47QPCh. 24 - Prob. 24.48QPCh. 24 - How many liters of air (78 percent N2, 22 percent...Ch. 24 - Prob. 24.50QPCh. 24 - Prob. 24.51QPCh. 24 - Prob. 24.52QPCh. 24 - Prob. 24.53QPCh. 24 - The combustion of 3.795 mg of liquid B, which...Ch. 24 - Prob. 24.55QPCh. 24 - Indicate the asymmetric carbon atoms in the...Ch. 24 - Prob. 24.57QPCh. 24 - Prob. 24.58QPCh. 24 - Prob. 24.59QPCh. 24 - Name the classes to which the following compounds...Ch. 24 - Prob. 24.61QPCh. 24 - Prob. 24.62QPCh. 24 - Prob. 24.63QPCh. 24 - Prob. 24.64QPCh. 24 - Prob. 24.65QPCh. 24 - Prob. 24.66QPCh. 24 - Prob. 24.67QPCh. 24 - When a mixture of methane and bromine vapor is...Ch. 24 - Prob. 24.69QPCh. 24 - Prob. 24.70QPCh. 24 - Prob. 24.71QPCh. 24 - Prob. 24.72QPCh. 24 - Prob. 24.73QPCh. 24 - Prob. 24.74QP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can I get help on drawing my arrowsarrow_forwardCan I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
