
Concept explainers
- a. The isoelectric point (pl) of phenylalanine is pH 5.5. Draw the structure of the major form of phenylalanine at pH values of 1, 5.5, and 11.
- b. The isoelectric point of histidine is pH 7 6. Draw the structures of the major forms of histidine at pH values of 1, 4, 7.6, and 11. Explain why the nitrogen in the histidine ring is a weaker base than the α-amino group.
- c. The isoelectric point of glutamic acid is pH 3.2. Draw the structures of the major forms of glutamic acid at pH values of 1,3.2, 7, and 11. Explain why the side-chain
carboxylic acid is a weaker acid than the acid group next to the α-carbon atom.
(a)
Interpretation:
The structures of the major form of phenylalanine at
Concept introduction:
The more acidic strength increases the formation of the hydrogen ions and decreases the
Answer to Problem 24.27SP
The structures of the major form of phenylalanine at
Explanation of Solution
The structures of the major forms of phenylalanine at
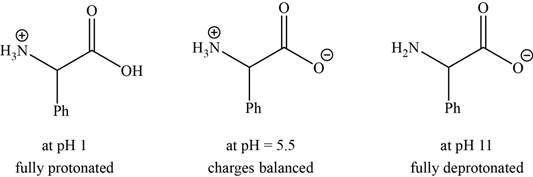
Figure 1
It is conferred from the above reaction that phenylalanine at
(b)
Interpretation:
The structures of the major forms of histidine at
Concept introduction:
The more acidic strength increases the formation of the hydrogen ions and decreases the
Answer to Problem 24.27SP
The structures of the major forms of histidine at
Explanation of Solution
The structures of the major forms of histidine at
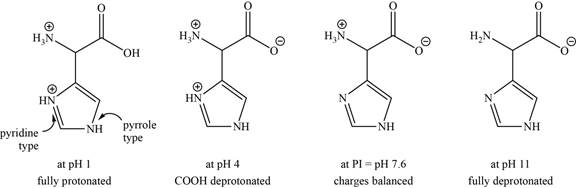
Figure 2
It is conferred from the above reaction that histidine at
(c)
Interpretation:
The structures of the major forms of glutamic acid at
Concept introduction:
The more acidic strength increases the formation of the hydrogen ions and decreases the
Answer to Problem 24.27SP
The structures of the major forms of glutamic acid at
Explanation of Solution
The structures of the major forms of glutamic acid at
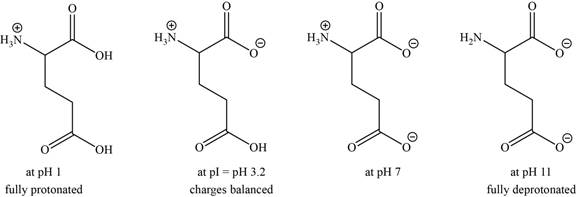
Figure 3
It is conferred from the above reaction that glutamic acid at
Want to see more full solutions like this?
Chapter 24 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
- Reagan is doing an atomic absorption experiment that requires a set of zinc standards in the 0.4-1.6 ppm range. A 1000 ppm Zn solution was prepared by dissolving the necessary amount of solid Zn(NO3)2 in water. The standards can be prepared by diluting the 1000 ppm Zn solution. Table 1 shows one possible set of serial dilutions (stepwise dilution of a solution) that Reagan could perform to make the necessary standards. Solution A was prepared by diluting 5.00 ml of the 1000 ppm Zn standard to 50.00 ml. Solutions C-E are called "calibration standards" because they will be used to calibrate the atomic absorption spectrometer. a. Compare the solution concentrations expressed as ppm Zn and ppm Zn(NO3)2. Compare the concentrations expressed as M Zn and M Zn(NO3)2 - Which units allow easy conversion between chemical species (e.g. Zn and Zn(NO3)2)? - Which units express concentrations in numbers with easily expressed magnitudes? - Suppose you have an analyte for which you don't know the molar…arrow_forwardNonearrow_forwardHow will you prepare the following buffers? 2.5 L of 1.5M buffer, pH = 10.5 from NH4Cl and NH3arrow_forward
- How will you prepare the following buffers? 2.5 L of 1.5M buffer, pH = 10.5 from NH4Cl and NH3arrow_forwardCH₂O and 22 NMR Solvent: CDCl3 IR Solvent: neat 4000 3000 2000 1500 1000 15 [ اند 6,5 9.8 3.0 7.0 6.0 5.0 4.8 3.0 2.0 1.0 9.8 200 100arrow_forwardprotons. Calculate the mass (in grams) of H3AsO4 (MW=141.9416) needed to produce 3.125 x 1026arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





