
Concept explainers
(a)
Interpretation: To identify the number of steps in glycolysis that consume ATP.
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:
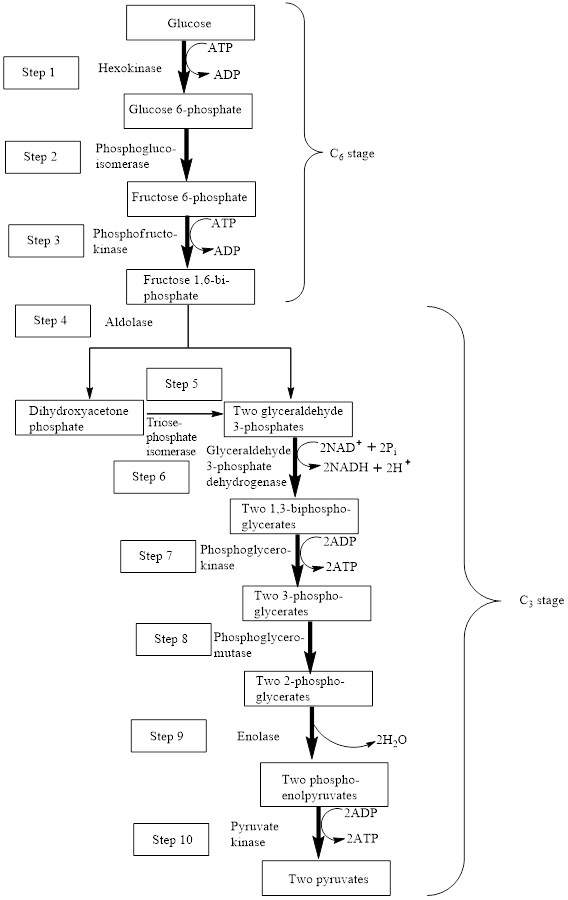
A reactant is defined as the substance that is initially present in the
Adenosine triphosphate (ATP) is a molecule that is defined as the energy currency of life and provides energy to carry out the metabolic processes in the living cells. It is converted either to adenosine monophosphate (AMP) or to adenosine diphosphate (ADP) after the consumption in the metabolic processes.
(b)
Interpretation: To identify the number of steps in glycolysis that involve oxidation.
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:

(c)
Interpretation: To identify the number of steps in glycolysis that involve NADH as a reactant.
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:

A reactant is defined as the substance that is initially present in the chemical reaction and gets consumed to form a new substance.
Nicotinamide adenine dinucleotide is associated with the
(d)
Interpretation: To identify the number of steps in glycolysis that involve a compound with a high-energy bond as a product.
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:

High energy compounds are those compounds that release a large amount of energy upon hydrolysis. These compounds consist of highly strained bonds that are responsible for the release of a high amount of energy. The compounds containing a phosphate group are examples of high energy compounds.
A high-energy phosphate group is formed when a phosphate group is attached to a carbon atom participating in carbon-oxygen or carbon-carbon double bond.
Trending nowThis is a popular solution!

Chapter 24 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- Determine how much ATP would a cell produce when using fermentation of a 50 mM glucose solution?arrow_forwardDetermine how much ATP would a cell produce when using aerobic respiration of a 7 mM glucose solution?arrow_forwardDetermine how much ATP would a cell produce when using aerobic respiration to degrade one small protein molecule into 12 molecules of malic acid, how many ATP would that cell make? Malic acid is an intermediate in the Krebs cycle. Assume there is no other carbon source and no acetyl-CoA.arrow_forward
- Identify each of the major endocrine glandsarrow_forwardCome up with a few questions and answers for umbrella species, keystone species, redunant species, and aquatic keystone speciesarrow_forward19. On the diagram below a. Label the three pictures as: DNA; polypeptide; or RNA. b. Label the arrows as: translation or transcription/RNA processing. c. Add the following details to the diagram. Promoter region TATA box Transcription start site Transcription terminator Intron (A,B,C,D) Exons (1,2,3,4,5) Splice sites 5' cap 5' UTR (untranslated region) 3' poly A tail 3' UTR (untranslated region) Translational start (AUG) Translational stop (UGA, UAG, or UAA) N and C ends of polypeptide 0000arrow_forward
- Match the letter labels in the figure below to the terms. Some letter labels are not used. MNNNNNNIN M C B A M D F E H K G 8arrow_forwardThe diagram below illustrates a quorum sensing pathway from Staphylococcus aureus. Please answer the following questions. 1. Autoinduction is part of the quorum sensing system. Which promoter (P2 or P3) is critical for autoinduction? 2)This staphylococcus aureus grows on human wounds, causing severe infections. You would like to start a clinical trial to treat these wound infections. Please describe: a) What molecule do you recommend for the trial. Why? b) Your trial requires that Staphylococcus aureus be isolated from the wound and submitted to genome sequencing before admittance. Why? What are you testing for? 3) If a mutation arises where the Promoter P3 is constitutively active, how would that influence sensitivity to AIP? Please explain your rationale. 4) This pathway is sensitive to bacterial cell density. Describe two separate mutation that would render the pathway active independent of cell density. Briefly explain your rationale. Mutation 1 Mutation 2arrow_forwardThere is currently a H5N1 cattle outbreak in North America. According to the CDC on Feb 26*: "A multistate outbreak of HPAI A(H5N1) bird flu in dairy cows was first reported on March 25, 2024. This is the first time that these bird flu viruses had been found in cows. In the United States, since 2022, USDA has reported HPAI A(H5N1) virus detections in more than 200 mammals." List and describe two mechanisms that could lead to this H5N1 influenza strain evolving to spread in human: Mechanisms 1: Mechanisms 2: For the mutations to results in a human epidered they would need to change how the virus interacts with the human host. In the case of mutations that may promote an epidemic, provide an example for: a protein that might incur a mutation: how the mutation would change interactions with cells in the respiratory tract (name the receptor on human cells) List two phenotypic consequence from this mutation that would increase human riskarrow_forward
- You have a bacterial strain with the CMU operon: a) As shown in the image below, the cmu operon encodes a peptide (Pep1), as well as a kinase and regulator corresponding to a two-component system. The cmu operon is activated when Pep 1 is added to the growth media. Pep1 is a peptide that when added extracellularly leads to activation of the Cmu operon. Pep1 cmu-kinase cmu-regulator You also have these genetic components in other strains: b) An alternative sigma factor, with a promoter activated by the cmu-regulator, that control a series of multiple operons that together encode a transformasome (cellular machinery for transformation). c) the gene cl (a repressor). d) the promoter X, which includes a cl binding site (and in the absence of cl is active). e) the gene gp (encoding a green fluorescence protein). Using the cmu operon as a starting point, and assuming you can perform cloning to rearrange any of these genomic features, how would you use one or more of these to modify the…arrow_forwardYou have identified a new species of a Gram-positive bacteria. You would like to screen their genome for all proteins that are covalently linked to the cell wall. You have annotated the genome, so that you identified all the promoters, operons, and genes sequences within the operons. Using these features, what would you screen for to identify a set of candidates for proteins covalently linked to the bacterial cell wall.arrow_forwardBelow is a diagram from a genomic locus of a bacterial genome. Each arrow represents a coding region, and the arrowheads indicate its orientation in the genome. The numbers are randomly assigned. Draw the following features on the diagram, and explain your rationale for each feature: 10 12 合會會會會長 6 a) Expected transcriptions, based on known properties of bacterial genes and operons. How many proteins are encoded in each of the transcripts? b) Location of promoters (include rationale) c) Location of transcriptional terminators (include rationale) d) Locations of Shine-Dalgarno sequences (include rationale)arrow_forward
 Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning





