
Concept explainers
(a)
Interpretation:
The preparation of given product from any two organohalides in which the organohalide can have no more than six carbon atoms should be identified.
Concept Introduction:
Preparation of organolithium: They are prepared by reaction of organohalide in presence of two equivalents of
Preparation of Grignard reagents: They are prepared by reaction of organohalide in presence
Reaction between Grignard reagent and water: Grignard reagents are strong bases which in presence of water forms hydrocarbon easily.
Gillman reagent: The lithium copper reagent compound
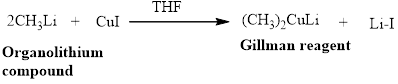
(b)
Interpretation:
The preparation of given product from any two organohalides in which the organohalide can have no more than six carbon atoms should be identified.
Concept Introduction:
Preparation of organolithium: They are prepared by reaction of organohalide in presence of two equivalents of
Preparation of Grignard reagents: They are prepared by reaction of organohalide in presence
Reaction between Grignard reagent and water: Grignard reagents are strong bases which in presence of water forms hydrocarbon easily.
Gillman reagent: The lithium copper reagent compound
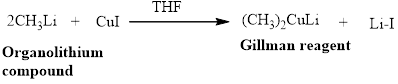
(c)
Interpretation:
The preparation of given product from any two organohalides in which the organohalide can have no more than six carbon atoms should be identified.
Concept Introduction:
Preparation of organolithium: They are prepared by reaction of organohalide in presence of two equivalents of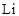 .
.
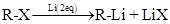
Preparation of Grignard reagents: They are prepared by reaction of organohalide in presence 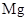 in ether solvent.
in ether solvent.
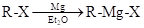
Reaction between Grignard reagent and water: Grignard reagents are strong bases which in presence of water forms hydrocarbon easily.
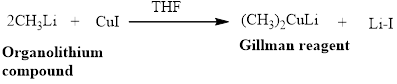
Gillman reagent: The lithium copper reagent compound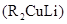 are very useful because unlike Grignard reagent and organolithium reagents, they react with organic halides to replace the halide group with an alkyl (R) group.
are very useful because unlike Grignard reagent and organolithium reagents, they react with organic halides to replace the halide group with an alkyl (R) group.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry, Third Edition Binder Ready Version
- 2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forwardComplete the spectroscopy with structurearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





