
(a)
Interpretation: A structure for the alkaloid halosaline, a reason for not getting the product in the given transformation in one step using LAH and a mechanism for the lactone-to-lactam ring contraction have to be found.
Concept Introduction:
Amides on reduction with lithium aluminum hydride followed by water work-up give the corresponding
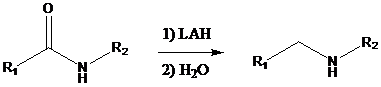
To find: Draw a structure for the alkaloid halosaline
Get the formation of lithium salt by reduction
(b)
Interpretation: A structure for the alkaloid halosaline, a reason for not getting the product in the given transformation in one step using LAH and a mechanism for the lactone-to-lactam ring contraction have to be found.
Concept Introduction:
If a compound contains both the azide and lactone ring, lithium aluminum hydride reduces both the groups, azide into the corresponding amine and the lactone ring into the
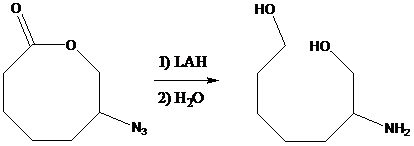
To find: Get the reason for achieving the given transformation in just one step (rather than two) using LAH to reduce both the azide and the amide in one reaction flask
Get the formation of lithium salt by reduction
(c)
Interpretation: A structure for the alkaloid halosaline, a reason for not getting the product in the given transformation in one step using LAH and a mechanism for the lactone-to-lactam ring contraction have to be found.
Concept Introduction:
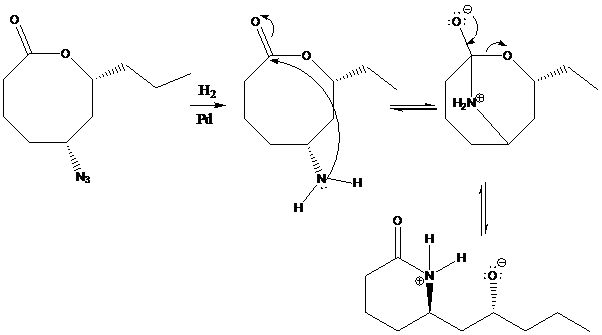
The simple lactone-to-lactam transformation occurs due to the driving force of amine nucleophiles. A nucleophile is a chemical species that donates an electron pair to an electrophile to form a
To find: Provide a mechanism for the lactone-to-lactam ring contraction
Provide the nucleophilic attack in the starting material
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry
- Provide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forward
- Indicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forwardIf I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forwardFeedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forwardIf I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





