
Concept explainers
(a)
Interpretation: The name and structure of the alcohols which on oxidation gives following product
Concept introduction:
Alcohols are the hydrocarbons which have
Alcohols can be of three types on the basis of degree of carbon atom to which the
Primary alcohol: The
Secondary alcohol: The
Tertiary alcohol: The
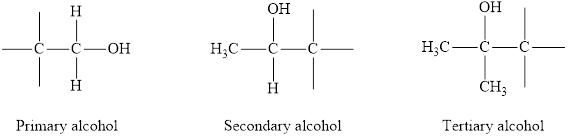
If a strong oxidizing agent is used, the oxidation of primary alcohol gives carboxylic acid.
A weaker oxidizing or partial oxidation of primary alcohol gives aldehydes.
While secondary alcohols always give ketones on oxidation.
(b)
Interpretation: The name and structure of the alcohols which on oxidation gives following product 2-hexanone
Concept introduction:
Alcohols are the hydrocarbons which have
Alcohols can be of three types on the basis of degree of carbon atom to which the
Primary alcohol: The
Secondary alcohol: The
Tertiary alcohol: The

If a strong oxidizing agent is used, the oxidation of primary alcohol gives
A weaker oxidizing or partial oxidation of primary alcohol gives aldehydes.
While secondary alcohols always give
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Chemistry and Chemical Reactivity - AP Edition
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning



