
Concept explainers
(a)
Interpretation: The theoretical yield of
Concept introduction:
Number of moles of a substance,
From its given mass is,
Theoretical yield is the maximum product yield that can be expected based on the masses of the reactants and the reaction stoichiometry.
2-Iodobenzoic acid is a crystaline solid and it can be prepared from 2-aminobenzoic acid treated with
The reaction can be represnted as follows,
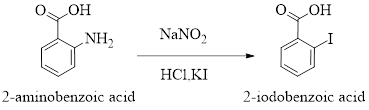
(a)
Answer to Problem 112IL
Theoretical yield of benzoic acid is
Explanation of Solution
From the given data,
Let’s calculate the number of moles of 2-amino benzoic acid:
From the given:
Let’s calculate the number of moles of
From the given:
Let’s calculate the number of moles of
From the equation , we can see that 1 mole of 2- amino benzoic acid gives 1 mole of 1-iodo benzoic acid .
For this conversion required 1 mole of
Theoretically produced
Molar mass of 2-iodobenzoic acid =
Therefore, theoretical yield of 2-iodobenzoic acid is
(b)
Interpretation: To Check the possibilty of isomers for 2-iodobenzoic acid
Concept introduction:
2-Iodobenzoic acid is a crystaline solid and it can be prepared from 2-aminobenzoic acid treated with
The reaction can be represnted as follows,
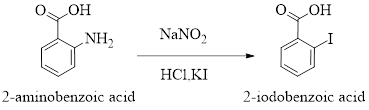
Two compounds that have the same molecular formula, but have different structural formulas are called isomers.
(b)
Answer to Problem 112IL
No other possible isomers for 2-Iodobenzoic acid.
Explanation of Solution
No other isomers of 2-iodo benzoic acid are possible in this reaction because the
(c)
Interpretation: The molar mass of the product formed in the given reaction has to be determined. And check whether the calculated molar mass is in reasonable agreement with the theoretical molar mass or not.
Concept introduction:
2-Iodobenzoic acid is a crystaline solid and it can be prepared from 2-aminobenzoic acid treated with
The reaction can be represnted as follows,
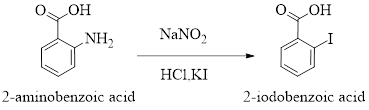
Concentration of solutions can be expressed in various terms; molarity is one such concentration expressing term.
Molarity (M) of a solution is the number of gram moles of a solute present in one liter of the solution.
A solution containing one gram mole or
Amount of substance (mol) can be determined by using the equation,
The molar mass of an element or compound is the mass in grams of 1 mole of that substance, and it is expressed in the unit of grams per mol (g/mol).
Theoretical yield is the maximum product yield that can be expected based on the masses of the reactants and the reaction stoichiometry.
(c)
Answer to Problem 112IL
Molar mass of 2-Iodobenzoic acid
There is only
Explanation of Solution
From the given,
Let’s calculate the number of mole of
Let’s calculate molar mass of 2-iodo benzoic acid.
Theoretical mass 2-Iodobenzoic acid is
Therefore, there is only
We can say that it is in reasonable agreement with theoretical molar mass.
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry and Chemical Reactivity - AP Edition
- Draw the monomers required to synthesize this condensation polymer.arrow_forward8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forward
- i need help on how to complete the followingarrow_forwardno AI walkthrough current image is wrong answerarrow_forwarda. Determine whether each of the Followery Molecules is in the R- On the y- Configuration 1-01"/ 1-6-4 Br 4 I el Br b. Draw The Fisher projection For all the Meso compounds that can exist FOR The Following molenlearrow_forward
- 1- Refer to the monosaccharides below to answer each of the following question(s): CH₂OH CHO CH₂OH CH₂OH 0 H- OH 0 0 HO- H H- -OH HO H HO H H OH HO- H CH₂OH H. OH HO H HO- H CH₂OH CH₂OH CH3 a. Sorbose b. Rhamnose c. Erythrulose d. Xylulose Classify each sugar by type; for example, glucose is an aldohexose. a. Xylulose is .. b. Erythrulose is . c. Sorbose is .. d. Rhamnose is .. 2- Consider the reaction below to answer the following question(s). CHO H OH CH₂OH CH₂OH HO- H HO HO + H. -OH HO OH HO. H OH OH H -OH H OH CH₂OH Q Z a. Refer to Exhibit 25-11. Place a triangle around the anomeric carbon in compound Q. Compound Z is: b. 1. the D-anomer. 2. the a-anomer. 3. the ẞ-anomer. 4. the L-anomer. c. Which anomer is the LEAST stable? d. Q and Z are cyclic examples of: a. acetals b. hemiacetals c. alditols d. hemialditolsarrow_forwardi need help identifying the four carbon oxygen bonds in the following:arrow_forwardImagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule HO H3N + The solution is... X O acidic OH O basic H3N-CH-C-O O neutral ○ (unknown) O acidic ○ basic CH2 CH 3-S-CH2 O neutral ○ (unknown) H3N O OH O acidic O basic Oneutral O (unknown) 0 H3N-CH-C-O CH3 CH CH3 O acidic O basic O neutral ○ (unknown) ? olo Ar BHarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



