
Organic Chemistry
3rd Edition
ISBN: 9781119338352
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 23, Problem 49PP
Interpretation Introduction
Interpretation:
The product formed in given reaction via Heck reaction and the reason for observed diastereoselectivity should be determined.
Concept Introduction:
Heck reaction: It describes the coupling reaction between aryl, vinyl or benzyl halide and
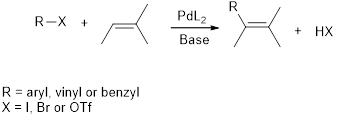
Stereoisomers are two compounds with the same molecular formula, but their orientation of atoms are different in space. Diastereomer is one type of stereoisomer.
Diastereomers: The chiral centers of two compounds give opposite configuration. These compounds do not have mirror images. Diastereoselectivity is formation of one diastereoisomer is preferred over the other one.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
-C = C - C - + Br₂ + I" -> -C-C-c
-C = C -C- + Br² + I₂
-C=C
Br I
+ Brū + Iz -7- C - C-C-
I Br
Mechanism;
- C = c - c - + Br - Br > - C-c-c-
Br
-C-C-C- + 1 - - -Ċ-Ċ'-c' -
Br
Br I
Write the mechanism of the esterification reaction (please show the mechanism included line pairs and arrows)
How do I break down the reaction shown on the chalkboard and explain it correctly using the bromonium ion mechanism, instead of the (disproven) carbocation-based mechanism
Chapter 23 Solutions
Organic Chemistry
Ch. 23.1 - Identify which of the following reagents is...Ch. 23.2 - Prob. 2CCCh. 23.2 - Prob. 3CCCh. 23.2 - Prob. 4CCCh. 23.2 - Prob. 5CCCh. 23.2 - Prob. 6CCCh. 23.3 - Show how you would prepare 1-butylcyclopentene...Ch. 23.3 - Prob. 7PTSCh. 23.3 -
Using any two organohalides of your choice...Ch. 23.3 - Prob. 9ATS
Ch. 23.4 - Prob. 10CCCh. 23.4 - Prob. 11CCCh. 23.5 - Prob. 2LTSCh. 23.6 - Prob. 3LTSCh. 23.6 - Prob. 16PTSCh. 23.6 - Prob. 17PTSCh. 23.6 - Prob. 18ATSCh. 23.6 - Prob. 19ATSCh. 23.7 - Prob. 4LTSCh. 23.7 - Prob. 20PTSCh. 23.7 - Prob. 21PTSCh. 23.7 - Prob. 22ATSCh. 23.7 - Prob. 23ATSCh. 23.8 - Prob. 5LTSCh. 23.8 - Prob. 24PTSCh. 23.8 - Prob. 25ATSCh. 23.8 - Prob. 26ATSCh. 23.9 - Prob. 27CCCh. 23.9 - Prob. 28CCCh. 23.9 - Prob. 29CCCh. 23.9 - Prob. 6LTSCh. 23.9 - Prob. 30PTSCh. 23.9 - Prob. 31PTSCh. 23.9 - Prob. 32ATSCh. 23 - Prob. 33PPCh. 23 - Prob. 34PPCh. 23 - Prob. 35PPCh. 23 - Prob. 36PPCh. 23 - Prob. 37PPCh. 23 - Prob. 38PPCh. 23 - Prob. 39PPCh. 23 - Prob. 40PPCh. 23 - Prob. 41PPCh. 23 - Prob. 42PPCh. 23 - Prob. 43PPCh. 23 - Prob. 44PPCh. 23 - Prob. 45PPCh. 23 - Prob. 46PPCh. 23 - Using 1-pentene as your only source of carbon...Ch. 23 - Prob. 48PPCh. 23 - Prob. 49PPCh. 23 - Prob. 50PPCh. 23 - Prob. 51PPCh. 23 - Prob. 52PPCh. 23 - Prob. 53PPCh. 23 - Prob. 54PPCh. 23 - Prob. 55PPCh. 23 - Prob. 56PPCh. 23 - Prob. 57PPCh. 23 - Prob. 58PPCh. 23 - Prob. 59IPCh. 23 - Prob. 60IPCh. 23 - Prob. 61IPCh. 23 - Prob. 62IPCh. 23 - Prob. 64IPCh. 23 - Prob. 66IPCh. 23 - Prob. 68IPCh. 23 - Prob. 69IPCh. 23 - Prob. 70IPCh. 23 - Prob. 71CPCh. 23 - Prob. 72CPCh. 23 - Prob. 73CPCh. 23 - Prob. 74CPCh. 23 - Prob. 75CPCh. 23 - Prob. 76CP
Knowledge Booster
Similar questions
- ¿Qué the product is obtained from tetraethoxypropano and hidrazina?. Indicate the reason why the corresponding dial is used.arrow_forwardIf CH3COCH2CH(OCH3)2 is reacted with hydrazine, two isomeric products are formed. Indicate their structures and the major product.arrow_forwardIs it possible to obtain addition derivatives to nitrogen in position 2 of pyrazoles by reaction with electrophilic agents? Reason for this.arrow_forward
- Starting from 1,3-dicarbonyl derivatives to obtain isooxazoles and isothiazoles. Indicate whether synthetic methods exist.arrow_forwardIn the synthesis of benzotriazole, adding NaNO2 heats the solution. State the reason.arrow_forwardIndicate the products obtained by treating benzotriazole with dimethyl sulfate or methyl iodide in a basic medium.arrow_forward
- What is the significance of selecting a "representative" sample for chemical analysis, and how does this practice ensure accurate and reliable results with respect to chemical analyses?arrow_forwardIdentify and provide an explanation of the differences between homogeneous and heterogeneous sampling in the context of sampling methods.arrow_forwardГ C-RSA CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0.0 b.092 0.797 1.088 1.813 C-RSA CHROMATOPAC CH=1 Report No. =13 ** CALCULATION REPORT ** DATA=1: @CHRM1.000 11/03/05 08:09:52 CH PKNO TIME 1 2 0.797 3 1.088 4 1.813 AREA 1508566 4625442 2180060 HEIGHT 207739 701206 V 287554 V MK IDNO CONC NAME 18.1447 55.6339 26.2213 TOTAL 8314067 1196500 100 C-R8A CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0. 0 087 337. 0.841 1.150 C-R8A CHROMATOPAC CH=1 Report No. =14 DATA=1: @CHRM1.000 11/03/05 08:12:40 ** CALCULATION REPORT ** CH PKNO TIME AREA 1 3 0.841 1099933 41.15 4039778 HEIGHT MK IDNO 170372 649997¯¯¯ CONC NAME 21.4007 78.5993 TOTAL 5139711 820369 100 3 C-R8A CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0.100 0:652 5.856 3 1.165 C-RSA CHROMATOPAC CH-1 Report No. =15 DATA=1: @CHRM1.000 11/03/05 08:15:26 ** CALCULATION REPORT ** CH PKNO TIME AREA HEIGHT MK IDNO CONC NAME 1 3 3 0.856 4 1.165 TOTAL 1253386 4838738 175481 708024 V 20.5739 79.4261 6092124…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY