
Concept explainers
(a)
Interpretation:
The monomer required to produce the given polymer needs to be determined.
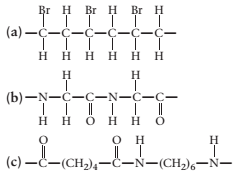
Concept Introduction:
A polymer is a long chain consists of large number of monomer units. The monomers are repeated units in a polymer they are linked to each other in different orders to produce different
These monomer units are linked to each other either through the formation of peptide linkage or glycosidic linkage or by removal of any moiety such as a water molecule.
Polyvinyl chloride, bakelite, polystyrene are some of the example of polymers.
(b)
Interpretation:
The monomer required to produce the given polymer needs to be determined.
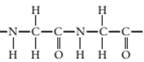
Concept Introduction:
A polymer is a long chain consists of large number of monomer units. The monomers are repeated units in a polymer they are linked to each other in different orders to produce different polymers.
These monomer units are linked to each other either through the formation of peptide linkage or glycosidic linkage or by removal of any moiety such as a water molecule.
Polyvinyl chloride, bakelite, polystyrene are some of the example of polymers.
(c)
Interpretation:
The monomer required to produce the given polymer needs to be determined.
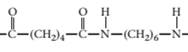
Concept Introduction:
A polymer is a long chain consists of large number of monomer units. The monomers are repeated units in a polymer they are linked to each other in different orders to produce different polymers.
These monomer units are linked to each other either through the formation of peptide linkage or glycosidic linkage or by removal of any moiety such as a water molecule.
Polyvinyl chloride, bakelite, polystyrene are some of the example of polymers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
PRINCIPLES+REACTIONS
- Use the expression below to ⚫ calculate its value and report it to the proper number of significant digits (you may need to round your answer). ⚫ calculate the % error (or % relative error or % inherent error) ⚫ calculate the absolute error. (20.54±0.02 × 0.254±0.003) / (3.21±0.05) = Value: % Error: Absolute error: ± | % (only 1 significant digit) (only 1 significant digit)arrow_forwardIn each case (more ductile, more brittle, more tough or resistant), indicate which parameter has a larger value. parameter Elastic limit Tensile strength more ductile Strain at break Strength Elastic modulus more fragile more tough or resistantarrow_forwardNonearrow_forward
- What functional groups are present in this IRarrow_forwardIn each case (more ductile, more brittle, more tough or resistant), indicate which parameter has a larger value. parameter Elastic limit Tensile strength more ductile Strain at break Strength Elastic modulus more fragile more tough or resistantarrow_forward4) A typical bottle of pop holds carbon dioxide at a pressure of 5 atm. What is the concentration of carbon dioxide in th solution? 5) A stream flowing over rocks and such is exposed to the atmosphere and well aerated. What would be the nitrogen concentration in the water at 25°C? (Air pressure is 1.000 bar.)arrow_forward
- Use the expression below to ⚫ calculate its value and report it to the proper number of significant digits (you may need to round your answer). ⚫ calculate the % error (or % relative error or % inherent error) ⚫ calculate the absolute error. (30.078±0.003) - (20.174±0.001) + (9.813±0.005) = Value: % Error: absolute error: ± % (only 1 significant digit) (only 1 significant digit)arrow_forwardDon't used Ai solution and don't used hand raitingarrow_forwardCircle the letter next to the most appropriate response. 1) Which is likely to be the least soluble with water? a) hexane b) acetone c) trichloromethane d) trinitro-toluene 2) Which is likely to be the most soluble in 3,4-dimethyloctane? a) hexane b) acetone c) trichloromethane d) trinitro-toluene 3) When ammonium nitrate is dissolved in water, the solution: a) gets warmer. b) gets colder. c) stays the same temperature. d) is none of the above because potassium nitrate is insoluble.arrow_forward
- Nonearrow_forwardCircle the compound below that you predict to be least soluble in water and explain yourselection. Please provide a throrough understanding.arrow_forwarditled [ The America | 241932100 交量 x Hanil Eco So | Question 5 ilearn.laccd.edu 0.5/0.5 pts How many amino acids do you see in the following structure? H3N-CH-C-N-CH-C-N-CH-C-N-CH-C-0- E-N-CH-E-N-CH-C-O- H₁C-CH | | H CH2 H CH₂ H CH2-C-NH2 CH3 CHANH, 6 ○ 5 3 4 H N 5 ptsarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





