
(a)
Interpretation:
The complete mechanism is to be drawn for the given reaction. The major product of the reaction is to be predicted.
Concept introduction:
Any substituents already present on the aromatic ring influence the position of substitution via their directing effect. Substituents that are electron-donating or weakly electron-withdrawing tend to direct the incoming electrophile to ortho-para positions. Substituents that are strongly electron-withdrawing tend to direct the incoming electrophile to meta positions.
In the case of substituted aromatic compounds containing more than one aromatic ring, the nature of existing substituents determines on which ring the further substitution will occur. An activating substuent will favor new substitution on the same ring while a deactivating substituent will favor new substitution on the other ring.
Electrophilic aromatic substitution on naphthalene generally favors substitution at the
Answer to Problem 23.65P
The complete mechanism for the given reaction is
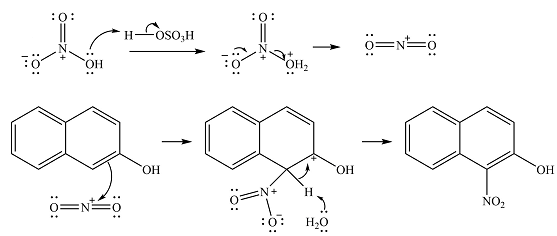
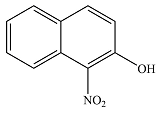
The major product of the reaction is
Explanation of Solution
The given reaction is
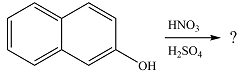
The substrate has two aromatic rings, with an OH substituent on one ring. This is an activating substituent, with a resonance electron-donating ability. The OH will, therefore, activate the ring it is attached to. Also, as an electron-donating substituent, it is ortho-para directing. The para position is blocked, so the substitution must occur at one of the two ortho positions. Substitution at the ortho position closer to the second ring (
The incoming electrophile is
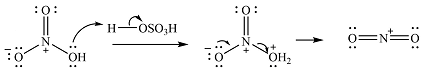
The electrophile will add to the carbon ortho to the OH group, adjacent to the second ring to form an arenium cation.
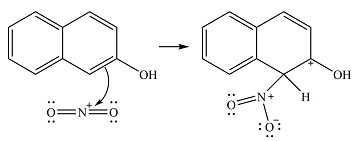
In the next step, a solvent molecule will extract the proton from that carbon. The
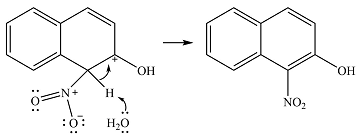
Therefore, the complete mechanism for the reaction can be drawn as

The major product of the reaction will be

The mechanism and major product of the given reaction were determined on the basis of the existing substuents and their activating/deactivating nature.
(b)
Interpretation:
The complete mechanism is to be drawn for the given reaction. The major product of the reaction is to be predicted.
Concept introduction:
Aromatic compounds undergo electrophilic aromatic substitution reactions. The reaction is actually an addition-elimination rather than direct substitution. The mechanism consists of the addition of the incoming electrophile to a ring carbon, producing an arenium cation, with a positive charge on an adjacent carbon. The second step is the elimination of the proton from the same carbon where the electrophile is attached. When alternate sites are available, the electrophile preferentially attaches to the position that produces the most stable arenium cation.
Any substituents already present on the aromatic ring influence the position of substitution via their directing effect. Substituents that are electron-donating or weakly electron-withdrawing tend to direct the incoming electrophile to ortho-para positions. Substituents that are strongly electron-withdrawing tend to direct the incoming electrophile to meta positions.
In the case of substituted aromatic compounds containing more than one aromatic ring, the nature of existing substituents determines on which ring the further substitution will occur. An activating substuent will favor new substitution on the same ring while a deactivating substituent will favor new substitution on the other ring.
Electrophilic aromatic substitution on naphthalene generally favors substitution at the
Answer to Problem 23.65P
The complete mechanism for the given reaction is
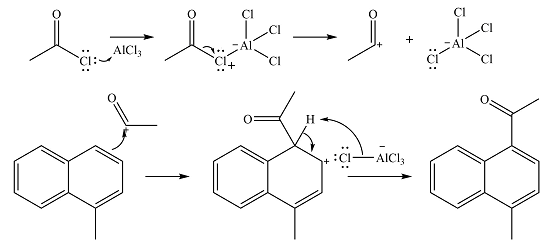
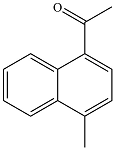
The major product of the reaction is
Explanation of Solution
The given reaction is
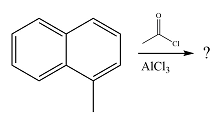
The methyl substituent already present is electron-donating and, therefore, an activating substituent. The substitution will occur on the same ring. Methyl group is ortho-para directing. One of the ortho positions is blocked, so substitution can occur at the other ortho position or the para position. The para position is an
The incoming electrophile is generated as a result of the coordination of the acyl chloride with the Lewis acid
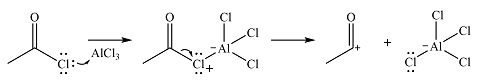
The electrophile will add to the
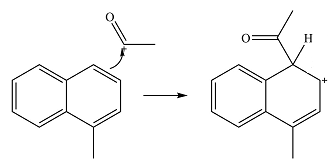
In the next step, the ion formed in the initial step will extract a proton from the
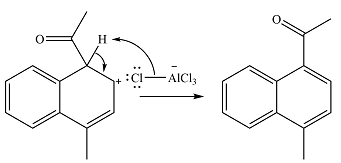
Thus, the complete mechanism for the reaction can be drawn as

And the major product will be

The mechanism and major product of the given reaction were determined on the basis of the existing substuents and their activating/deactivating nature.
(c)
Interpretation:
The complete mechanism is to be drawn for the given reaction. The major product of the reaction is to be predicted.
Concept introduction:
Aromatic compounds undergo electrophilic aromatic substitution reactions. The reaction is actually an addition-elimination rather than direct substitution. The mechanism consists of the addition of the incoming electrophile to a ring carbon, producing an arenium cation, with a positive charge on an adjacent carbon. The second step is the elimination of the proton from the same carbon where the electrophile is attached. When alternate sites are available, the electrophile preferentially attaches to the position that produces the most stable arenium cation.
Any substituents already present on the aromatic ring influence the position of substitution via their directing effect. Substituents that are electron-donating or weakly electron-withdrawing tend to direct the incoming electrophile to ortho-para positions. Substituents that are strongly electron-withdrawing tend to direct the incoming electrophile to meta positions.
In the case of substituted aromatic compounds containing more than one aromatic ring, the nature of existing substituents determines on which ring the further substitution will occur. An activating substuent will favor new substitution on the same ring while a deactivating substituent will favor new substitution on the other ring.
Electrophilic aromatic substitution on naphthalene generally favors substitution at the
Answer to Problem 23.65P
The complete mechanism of the reaction is
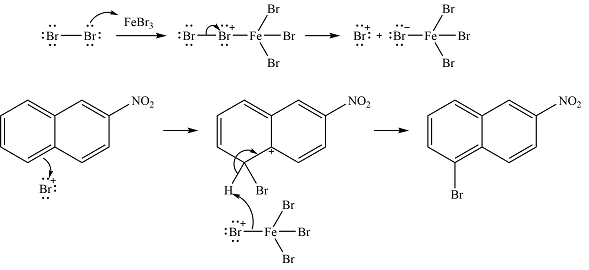
The major product is
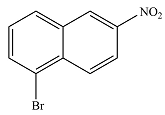
Explanation of Solution
The given reaction is
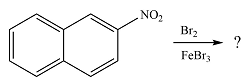
The nitro group already present is strongly electron-withdrawing, and therefore, a deactivating substituent. The substitution will occur on the other ring. On this ring, substitution at the
The incoming electrophile is generated as a result of the coordination of the bromine molecule with the Lewis acid
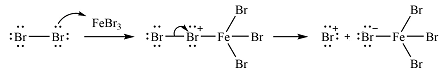
The electrophile will add to the
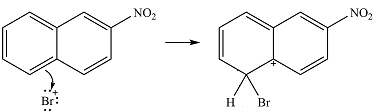
In the next step, the
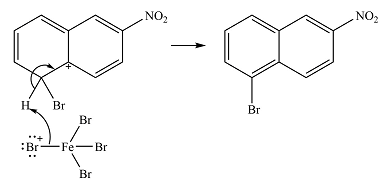
Thus, the complete mechanism can be drawn as

The major product will be

The mechanism and major product of the given reaction were determined on the basis of the existing substuents and their activating/deactivating nature.
(d)
Interpretation:
The complete mechanism is to be drawn for the given reaction. The major product of the reaction is to be predicted.
Concept introduction:
Aromatic compounds undergo electrophilic aromatic substitution reactions. The reaction is actually an addition-elimination rather than direct substitution. The mechanism consists of the addition of the incoming electrophile to a ring carbon, producing an arenium cation, with a positive charge on an adjacent carbon. The second step is the elimination of the proton from the same carbon where the electrophile is attached. When alternate sites are available, the electrophile preferentially attaches to the position that produces the most stable arenium cation.
Any substituents already present on the aromatic ring influence the position of substitution via their directing effect. Substituents that are electron-donating or weakly electron-withdrawing tend to direct the incoming electrophile to ortho-para positions. Substituents that are strongly electron-withdrawing tend to direct the incoming electrophile to meta positions.
In the case of substituted aromatic compounds containing more than one aromatic ring, the nature of existing substituents determines on which ring the further substitution will occur. An activating substuent will favor new substitution on the same ring while a deactivating substituent will favor new substitution on the other ring.
Electrophilic aromatic substitution on naphthalene generally favors substitution at the
Answer to Problem 23.65P
The complete mechanism for the given reaction is
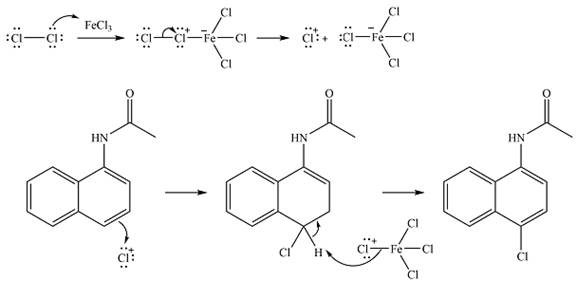
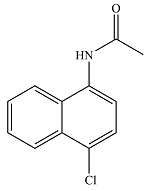
The major product of the reaction is
Explanation of Solution
The given reaction is
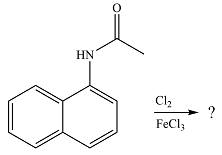
The substituent already present is activating as the nitrogen can donate electrons to the ring. The substitution will, therefore, occur on the same ring. The electron-donating substituent is ortho-para directing. One of the ortho positions is aready blocked. Out of the remaining ortho position and the para position, a substitution at the para position will be favored as this is also the
The elecrophile is generated as a result of the coordination of the chlorine molecule with the Lewis acid
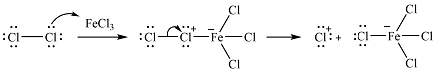
The electrophile will add to the
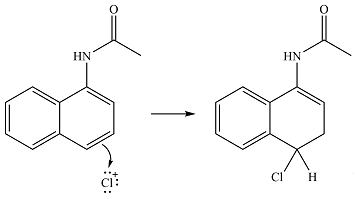
In the next step,
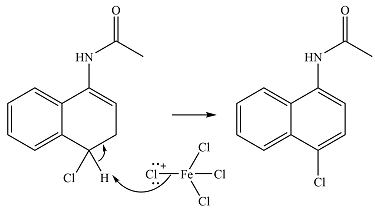
Thus, the complete mechanism for the reaction can be drawn as

And the major product of the reaction will be

The mechanism and major product of the given reaction were determined on the basis of the existing substuents and their activating/deactivating nature.
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry: Principles And Mechanisms
- Draw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forwardDraw the monomers required to synthesize this condensation polymer please.arrow_forward
- Provide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forwardIdentify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forward
- H- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forwardWhy doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward6. A solution (0.0004 M) of Fe(S2CNEt2)3 (see the structural drawing below) in chloroform has absorption bands at: 350 nm (absorbance A = 2.34); 514 nm(absorbance A = 0.0532); Calculate the molar absorptivity values for these bands. Comment on their possible nature (charge transfer transitions or d-d S N- transitions?). (4 points)arrow_forward
- What is the mechanism for this?arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward7. Describe the expected 31P and 19F (where applicable) NMR spectral patterns for the following compounds (indicate number of signals and their splitting patterns). a) tetraphenyldiphosphine Ph Ph P-P Ph Ph Ph Ph ' b) tetraphenyldiphosphine monoxide P-P-Ph Ph (2 points) (2 points c) tetrafluorophosphonium hexafluorophosphate [PF4]*[PF6]¯ (4 points)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





