
(a)
Interpretation:
The major product with detailed mechanism for the given reaction is to be drawn.
Concept introduction:
The nitration is the electrophilic aromatic substitution reaction. The aromatic ring on reaction with nitric acid or with a mixture of concentrated nitric acid and sulfuric acid undergoes substitution of one of the ring hydrogen by nitro group and forms nitrobenzene, this is called nitration. The electron withdrawing groups deactivates the aromatic ring and decreases the electron density at ortho-para position comparatively than meta position. Thus they are meta directors. That means electrophilic aromatic substitution preferably occurs at meta position.
Answer to Problem 23.34P
The major product with detailed mechanism for the given reaction is:
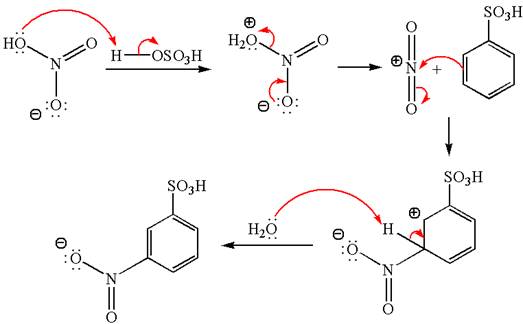
Explanation of Solution
The given equation is:
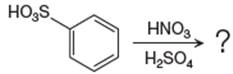
In this
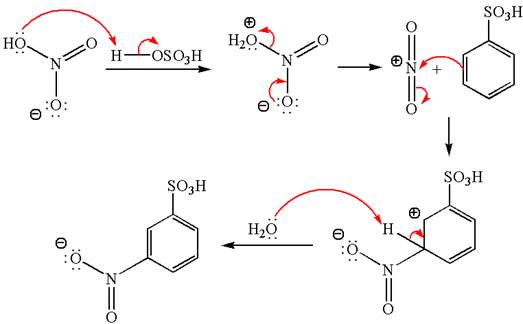
The product with detailed mechanism is drawn by identifying the substituent at ring is meta director and on nitration introduced nitro group to ring.
(b)
Interpretation:
The products with detailed mechanisms for the formation of each product are to be drawn.
Concept introduction:
The chlorination of aromatic ring can be carried out by reacting with
Answer to Problem 23.34P
The products with detailed mechanisms for the formation of each product are:
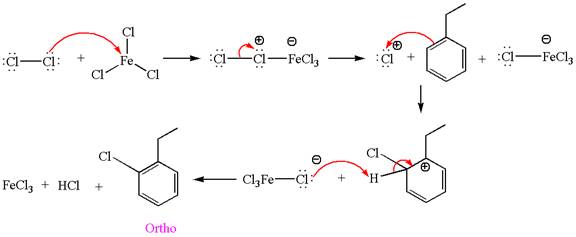
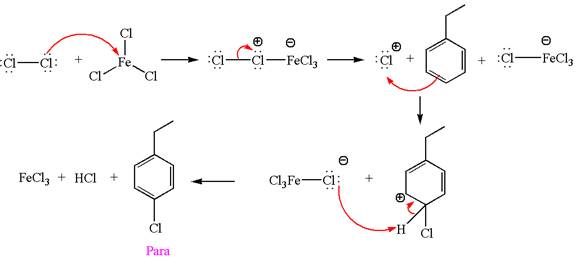
Explanation of Solution
The given equation is:
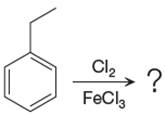
In this aromatic compound, the benzene ring has ethyl group attached. The ethyl group is electron donating inductively and activates the ring at ortho-para positions. Thus it is ortho-para director, on chlorination with
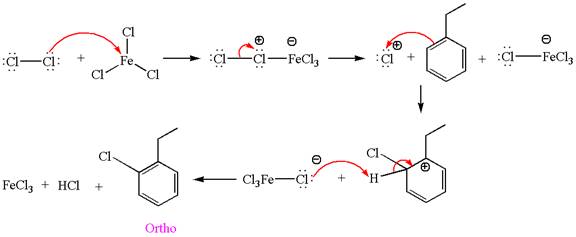
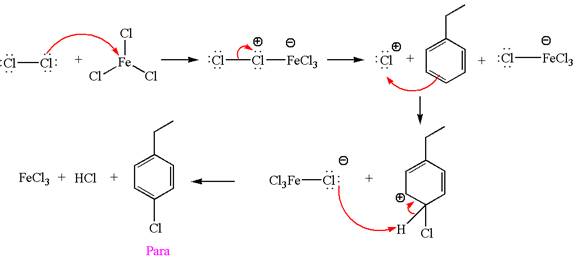
The products with detailed mechanisms are drawn by identifying the substituent at ring is ortho-para director and on chlorination introduced chlorine atom to ring.
(c)
Interpretation:
The products with detailed mechanisms for the formation of each product are to be drawn.
Concept introduction:
The aromatic ring on reaction with acyl chloride in
Answer to Problem 23.34P
The products with detailed mechanisms for the formation of each product are:
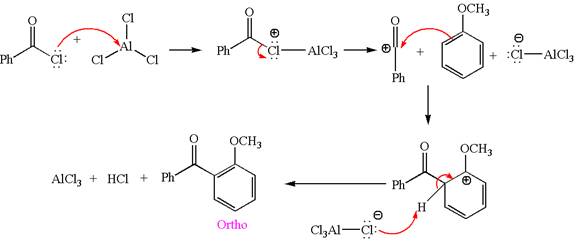
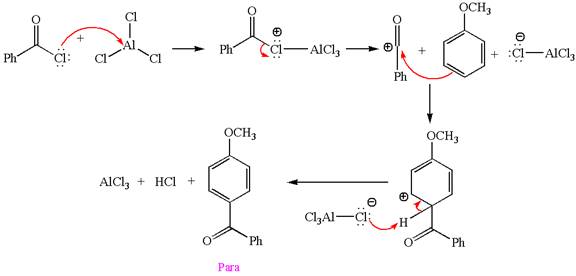
Explanation of Solution
The given equation is:
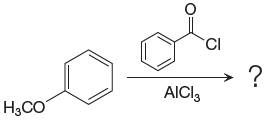
In this aromatic compound, the benzene ring has methoxy group attached. The methoxy group is electron donating and activates the ring at ortho-para positions. Thus it is ortho-para director, on Friedel-Craft acylation in presence of
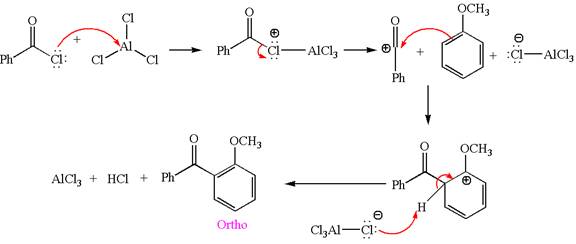
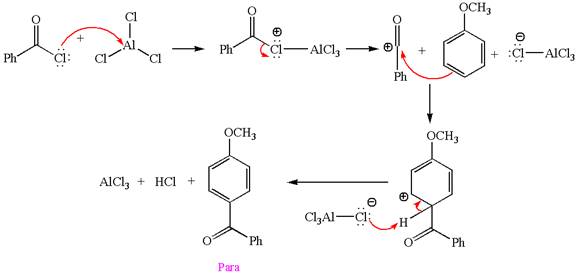
The products with detailed mechanisms are drawn by identifying the substituent at ring is ortho-para director and on F.C. acylation introduced acyl group to ring.
(d)
Interpretation:
The major product with detailed mechanism for the given reaction is to be drawn.
Concept introduction:
The bromination of aromatic ring can be carried out by reacting with
Answer to Problem 23.34P
The major product with detailed mechanism for the given reaction is:
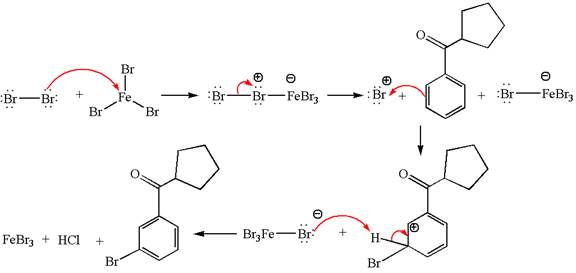
Explanation of Solution
The given equation is:
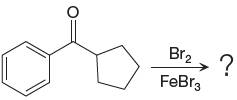
In this aromatic compound, the benzene ring has carbonyl group attached. The carbonyl group is electron withdrawing and deactivates the ring at ortho-para positions. Thus it is meta director, on bromination with

The product with detailed mechanism is drawn by identifying the substituent at ring is meta director and on bromination introduced bromine atom to ring.
(e)
Interpretation:
The products with detailed mechanisms for the formation of each product are to be drawn.
Concept introduction:
The nitration is the electrophilic aromatic substitution reaction. The aromatic ring on reaction with nitric acid or with a mixture of concentrated nitric acid and sulfuric acid undergoes substitution of one of the ring hydrogen by nitro group and forms nitrobenzene, this is called nitration. The electron donating groups activates the aromatic ring and increases the electron density at ortho-para position comparatively than meta position. That means electrophilic aromatic substitution preferably occurs at ortho-para position.
Answer to Problem 23.34P
The products with detailed mechanisms for the formation of each product are:
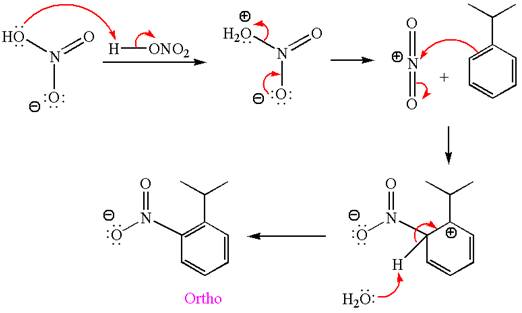
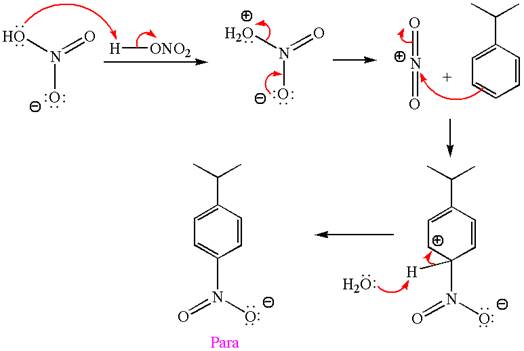
Explanation of Solution
The given equation is:
In this aromatic compound, the benzene ring has methoxy group attached. The methoxy group is electron donating and activates the ring at ortho-para positions. Thus it is ortho-para director, on nitration using
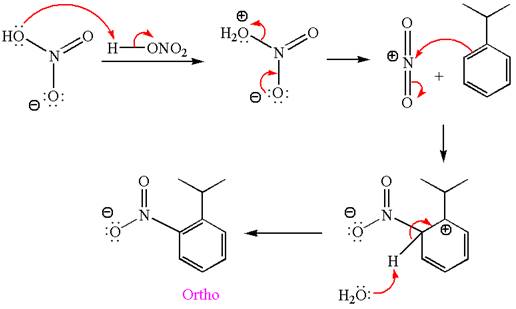
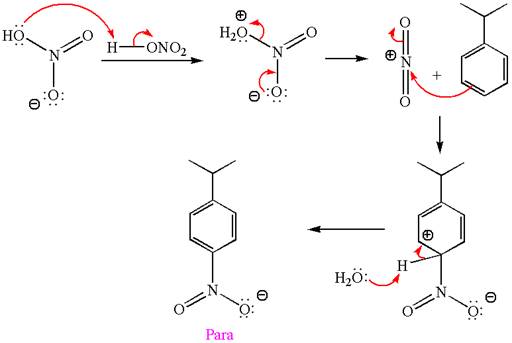
The products with detailed mechanisms are drawn by identifying the substituent at ring is ortho-para director and on nitration introduced nitro group to ring.
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry: Principles And Mechanisms
- Draw a tetramer of this alternating copolymer.arrow_forwardH I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forwardPolylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forward
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





