
(a)
Interpretation:
The isomer from the given pair that will undergo electrophilic
Concept introduction:
The electron donating groups activate whereas the electron withdrawing groups deactivate the aromatic ring. The electron donating groups are ortho-para directing, which means electrophilic aromatic substitution preferably occurs at the ortho-para position.
Answer to Problem 23.46P
The
Explanation of Solution
The given pair is
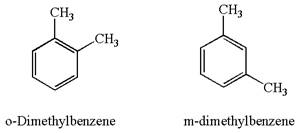
In both structures, benzene has two methyl substituents, which are activating groups. They activate the ring at ortho-para direction. As the position of methyl groups is different in these structures, their activating position on the ring would be different. In both the structures, each methyl activates the ring at two positions, as shown below:
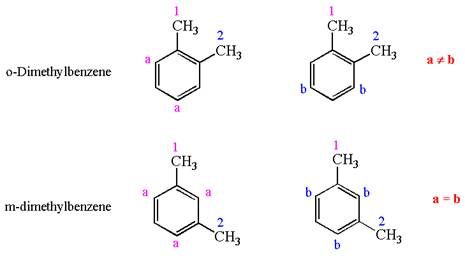
The activating positions of first methyl are indicated by letter
It is determined that
(b)
Interpretation:
The isomer from the given pair that will undergo electrophilic aromatic substitution faster is to be determined.
Concept introduction:
The electron donating groups activate whereas the electron withdrawing groups deactivate the aromatic ring. The electron donating groups are ortho-para directing, which means electrophilic aromatic substitution preferably occurs at the ortho-para position.
Answer to Problem 23.46P
The
Explanation of Solution
The given pair is
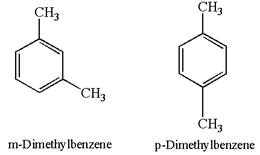
In both structures, the benzene has two methyl substituents, which are activating groups. They activate the ring at ortho-para direction. As the position of methyl groups is different in these structures, their activating position on ring would be different. In both the structures, each methyl activates the ring at two positions, as shown below:
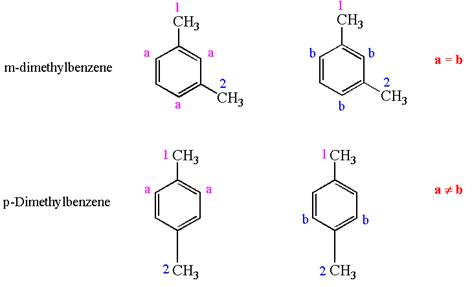
The activating positions of first methyl are indicated by letter
It is determined that
(c)
Interpretation:
The isomer from the given pair that will undergo electrophilic aromatic substitution faster is to be determined.
Concept introduction:
The electron donating groups activate whereas the electron withdrawing groups deactivate the aromatic ring. The electron donating groups are ortho-para directing, which means electrophilic aromatic substitution preferably occurs at the ortho-para position.
Answer to Problem 23.46P
The
Explanation of Solution
The given pair is
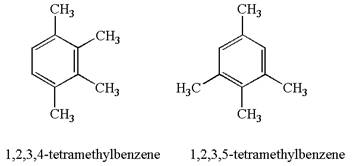
In both structures, the benzene has four methyl substituents, which are activating groups. They activate the ring at ortho-para direction. As the position of methyl groups is different in these structures, their activating position on ring would be different. In both the structures, each methyl activates the ring at two positions, as shown below:
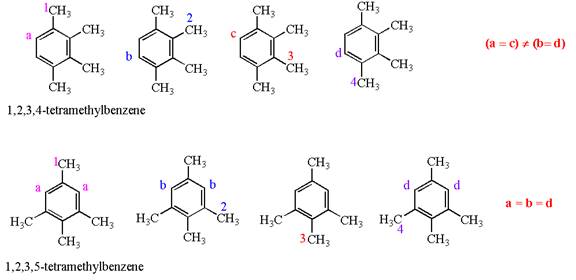
The activating positions of first methyl are indicated by letter
In case of
The methyl groups in
It is determined that
Want to see more full solutions like this?
Chapter 23 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- 3. a. Use the MS to propose at least two possible molecular formulas. For an unknown compound: 101. 27.0 29.0 41.0 50.0 52.0 55.0 57.0 100 57.5 58.0 58.5 62.0 63.0 64.0 65.0 74.0 40 75.0 76.0 20 20 40 60 80 100 120 140 160 180 200 220 m/z 99.5 68564810898409581251883040 115.0 116.0 77404799 17417M 117.0 12.9 118.0 33.5 119.0 36 133 0 1.2 157.0 2.1 159.0 16 169.0 219 170.0 17 171.0 21.6 172.0 17 181.0 1.3 183.0 197.0 100.0 198.0 200. 784 Relative Intensity 2 2 8 ō (ppm) 6 2arrow_forwardSolve the structure and assign each of the following spectra (IR and C-NMR)arrow_forward1. For an unknown compound with a molecular formula of C8H100: a. What is the DU? (show your work) b. Solve the structure and assign each of the following spectra. 8 6 2 ō (ppm) 4 2 0 200 150 100 50 ō (ppm) LOD D 4000 3000 2000 1500 1000 500 HAVENUMBERI -11arrow_forward
- 16. The proton NMR spectral information shown in this problem is for a compound with formula CioH,N. Expansions are shown for the region from 8.7 to 7.0 ppm. The normal carbon-13 spec- tral results, including DEPT-135 and DEPT-90 results, are tabulated: 7 J Normal Carbon DEPT-135 DEPT-90 19 ppm Positive No peak 122 Positive Positive cus и 124 Positive Positive 126 Positive Positive 128 No peak No peak 4° 129 Positive Positive 130 Positive Positive (144 No peak No peak 148 No peak No peak 150 Positive Positive してしarrow_forward3. Propose a synthesis for the following transformation. Do not draw an arrow-pushing mechanism below, but make sure to draw the product of each proposed step (3 points). + En CN CNarrow_forwardShow work..don't give Ai generated solution...arrow_forward
- Label the spectrum with spectroscopyarrow_forwardQ1: Draw the most stable and the least stable Newman projections about the C2-C3 bond for each of the following isomers (A-C). Are the barriers to rotation identical for enantiomers A and B? How about the diastereomers (A versus C or B versus C)? enantiomers H Br H Br (S) CH3 H3C (S) (R) CH3 H3C H Br A Br H C H Br H3C (R) B (R)CH3 H Br H Br H3C (R) (S) CH3 Br H D identicalarrow_forwardLabel the spectrumarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





