
(a)
Interpretation: To classify each of the following molecules as (1) an oxidizing agent, (2) a reducing agent, or (3) neither an oxidizing agent nor a reducing agent.
a. NADH
b. ATP
c. FAD
d. CoA–SH
Concept introduction: The sum of various
ATP is a
Flavin adenine dinucleotideexists in two forms: oxidized form
Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation.
Oxidizing agents are those species which gets reduced and oxidizes the other species present in the chemical reaction. Reducing agent is those species which gets oxidized and reduces the other species present in a chemical reaction. Generally, oxidizing agents are electron acceptor and reducing agents are electron donor.
(a)
Answer to Problem 23.44EP
Explanation of Solution
Nicotinamide adenine dinucleotide exists in two forms:
Here
(b)
Interpretation: To classify each of the following molecules as (1) an oxidizing agent, (2) a reducing agent, or (3) neither an oxidizing agent nor a reducing agent.
a. NADH
b. ATP
c. FAD
d. CoA–SH
Concept introduction: The sum of various chemical reactions occurring in the human body is called metabolism and the reactions individually are known as metabolic reactions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions.ATP,
ATP is a nucleotide which provides energy for the completion of various metabolic reactions occurring in our human body. The structure of ATP consists of adenine base, ribose sugar unit and the three phosphate groupconnected to each other by phosphoanhydride bonds.
Flavin adenine dinucleotideexists in two forms: oxidized form
Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation.
Oxidizing agents are those species which gets reduced and oxidizes the other species present in the chemical reaction. Reducing agent is those species which gets oxidized and reduces the other species present in a chemical reaction. Generally, oxidizing agents are electron acceptor and reducing agents are electron donor.
(b)
Answer to Problem 23.44EP
ATP molecule is neither a reducing agent nor an oxidizing agent in metabolic reactions.
Explanation of Solution
Adenosine triphosphate (ATP) is anucleotide which structural component is one unit of the adenine base, one unit of ribose sugar and three units of a phosphate group. It can be converted into its monophosphate form(AMP) and diphosphate form(ADP) by losing a phosphate group. The reaction to this change is:
Here ATP is not involved in electron transfer hence it is neither a reducing agent nor an oxidizing agent.
(c)
Interpretation: To classify each of the following molecules as (1) an oxidizing agent, (2) a reducing agent, or (3) neither an oxidizing agent nor a reducing agent.
a. NADH
b. ATP
c. FAD
d. CoA–SH
Concept introduction: The sum of various chemical reactions occurring in the human body is called metabolism and the reactions individually are known as metabolic reactions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions.ATP,
ATP is a nucleotide which provides energy for the completion of various metabolic reactions occurring in our human body. The structure of ATP consists of adenine base, ribose sugar unit and the three phosphate groupconnected to each other by phosphoanhydride bonds.
Flavin adenine dinucleotideexists in two forms: oxidized form
Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation.
Oxidizing agents are those species which gets reduced and oxidizes the other species present in the chemical reaction. Reducing agent is those species which gets oxidized and reduces the other species present in a chemical reaction. Generally, oxidizing agents are electron acceptor and reducing agents are electron donor.
(c)
Answer to Problem 23.44EP
Explanation of Solution
Flavin adenine dinucleotide exists in two forms:
Here
(d)
Interpretation: To classify each of the following molecules as (1) an oxidizing agent, (2) a reducing agent, or (3) neither an oxidizing agent nor a reducing agent.
a. NADH
b. ATP
c. FAD
d. CoA–SH
Concept introduction: The sum of various chemical reactions occurring in the human body is called metabolism and the reactions individually are known as metabolic reactions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions.ATP,
ATP is a nucleotide which provides energy for the completion of various metabolic reactions occurring in our human body. The structure of ATP consists of adenine base, ribose sugar unit and the three phosphate groupconnected to each other by phosphoanhydride bonds.
Flavin adenine dinucleotideexists in two forms: oxidized form
Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation.
Oxidizing agents are those species which gets reduced and oxidizes the other species present in the chemical reaction. Reducing agent is those species which gets oxidized and reduces the other species present in a chemical reaction. Generally, oxidizing agents are electron acceptor and reducing agents are electron donor.
(d)
Answer to Problem 23.44EP
Coenzyme A (CoA–SH) molecule is neither a reducing agent nor an oxidizing agent in metabolic reactions.
Explanation of Solution
Coenzyme A (CoA) is a coenzyme whose structure is based on the B vitamin pantothenic acid. Its structure consists of three subunits: 2-Aminoethanethiol, pantothenic acid, and phosphorylated ADP.
Coenzyme A is always in equilibrium with its acetyl form and therefore helps in transfer of acetyl group in metabolic reaction. The reaction for this change is
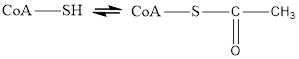
Here Coenzyme A (CoA) is not involved in electron transfer hence it is neithera reducing agent nor an oxidizing agent.
a.
b. ATP molecule is neither a reducing agent nor an oxidizing agent in metabolic reactions.
c.
d. Coenzyme A (CoA–SH) molecule is neither a reducing agent nor an oxidizing agent in metabolic reactions.
Want to see more full solutions like this?
Chapter 23 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- Reaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward
- 8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forward
- о но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forward
- If I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





