
Concept explainers
(a)
Interpretation:
The product obtained in the reaction of
Concept introduction:
Answer to Problem 23.44AP
The product obtained in the reaction of
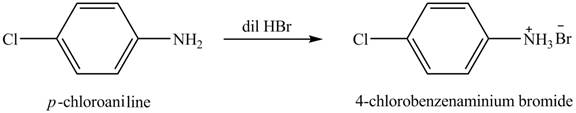
Explanation of Solution
When
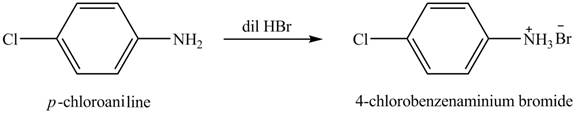
Figure 1
The product obtained in the reaction of
(b)
Interpretation:
The product obtained in the reaction of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. When alcohol reacts with hydrogen halide it forms
Answer to Problem 23.44AP
The product obtained in the reaction of
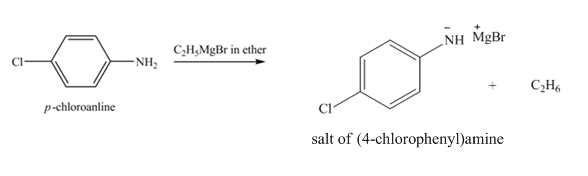
Explanation of Solution
The reaction of

Figure 2
The product obtained in the reaction of
(c)
Interpretation:
The product obtained in the reaction of
Concept introduction:
The formation of diazonium salt from
Answer to Problem 23.44AP
The product obtained in the reaction of
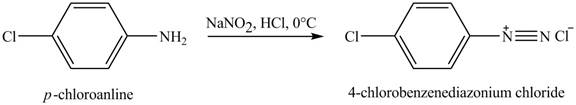
Explanation of Solution
When

Figure 3
The product obtained in the reaction of
(d)
Interpretation:
The product obtained in the reaction of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.44AP
The product
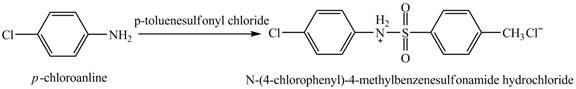
Explanation of Solution
When

Figure 4
The product
(e)
Interpretation:
The product obtained in the reaction of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. The formation of diazonium salt from aromatic amines takes place using sodium nitrite and hydrochloric acid at low temperatures. Aryl diazonium salts undergo a variety of specific substitution reactions in which the incoming Z group replaces
Answer to Problem 23.44AP
The product
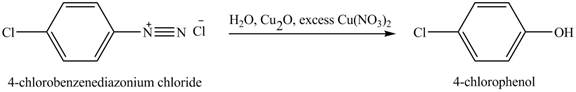
Explanation of Solution
When
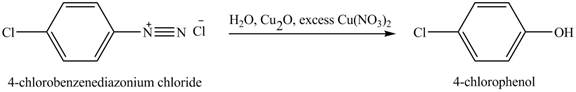
Figure 5
The product obtained in the reaction of
(f)
Interpretation:
The product obtained in the reaction of
Concept introduction:
The formation of diazonium salt from aromatic amines takes place using sodium nitrite and hydrochloric acid at low temperatures. Aryl diazonium salts undergo a variety of specific substitution reactions in which the incoming Z group replaces
Answer to Problem 23.44AP
The product
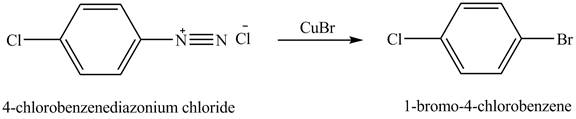
Explanation of Solution
When
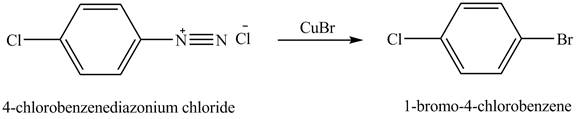
Figure 6
The product
(g)
Interpretation:
The product obtained in the reaction of
Concept introduction:
The formation of diazonium salt from aromatic amines takes place using sodium nitrite and hydrochloric acid at low temperatures. Aryl diazonium salts undergo a variety of specific substitution reactions in which the incoming Z group replaces
Answer to Problem 23.44AP
The product chlorobenzene is obtained in the reaction of the product of part (c) and
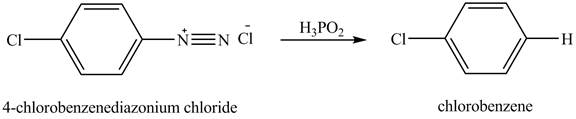
Explanation of Solution
The reduction reaction of
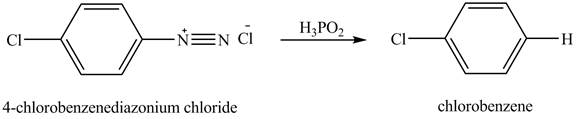
Figure 7
The product chlorobenzene is obtained in the reaction of the product of part (c) and
(h)
Interpretation:
The product obtained in the reaction of
Concept introduction:
The formation of diazonium salt from aromatic amines takes place using sodium nitrite and hydrochloric acid at low temperatures. Aryl diazonium salts undergo a variety of specific substitution reactions in which the incoming Z group replaces
Answer to Problem 23.44AP
The product,
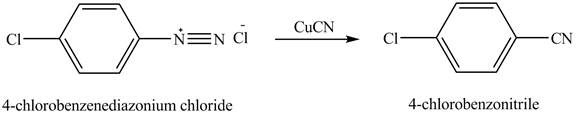
Explanation of Solution
When

Figure 8
The product,
(i)
Interpretation:
The product obtained in the reaction of
Concept introduction:
The formation of diazonium salt from aromatic amines takes place using sodium nitrite and hydrochloric acid at low temperatures. Aryl diazonium salts undergo a variety of specific substitution reactions in which the incoming Z group replaces N2 (a very good leaving group) to form corresponding products.
Answer to Problem 23.44AP
The product
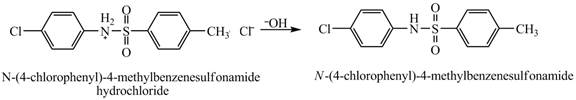
Explanation of Solution
When

Figure 9
The product
Want to see more full solutions like this?
Chapter 23 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Draw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forward
- Write the aldol condensation mechanism and product for benzaldehyde + cyclohexanone in a base. Then trans-cinnamaldehyde + acetone in base. Then, trans-cinnamaldehyde + cyclohexanone in a base.arrow_forwardClick the "draw structure" button to launch the drawing utility. Draw the structure of the alkene that yields the following set of oxidative cleavage products? draw structure ...arrow_forwardWrite the mechanism for the reaction.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


