
(a)
Interpretation:
The major product with detailed mechanism for the given reaction is to be drawn.
Concept introduction:
The nitration is the electrophilic aromatic substitution reaction. The aromatic ring on reaction with nitric acid or with a mixture of concentrated nitric acid and sulfuric acid undergoes substitution of one of the ring hydrogen by nitro group and forms nitrobenzene, this is called nitration. The electron withdrawing groups deactivates the aromatic ring and decreases the electron density at ortho-para position comparatively than meta position. Thus they are meta directors. That means electrophilic aromatic substitution preferably occurs at meta position.
Answer to Problem 23.34P
The major product with detailed mechanism for the given reaction is:
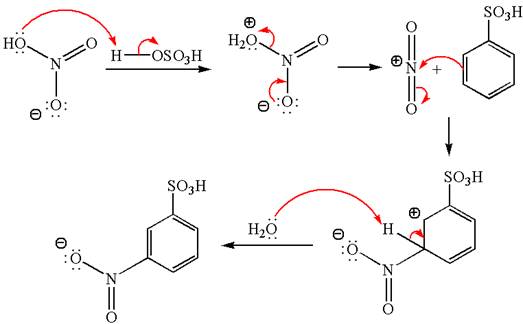
Explanation of Solution
The given equation is:
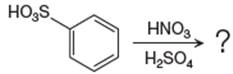
In this
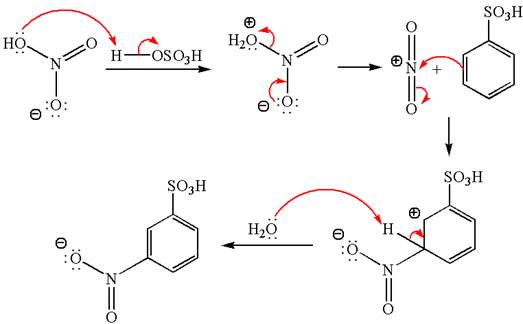
The product with detailed mechanism is drawn by identifying the substituent at ring is meta director and on nitration introduced nitro group to ring.
(b)
Interpretation:
The products with detailed mechanisms for the formation of each product are to be drawn.
Concept introduction:
The chlorination of aromatic ring can be carried out by reacting with
Answer to Problem 23.34P
The products with detailed mechanisms for the formation of each product are:
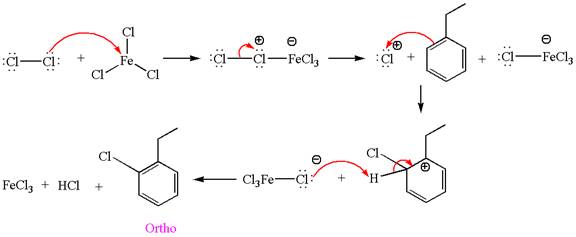
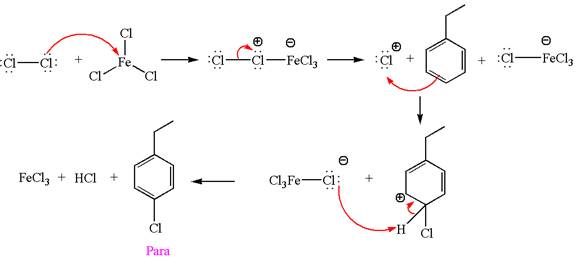
Explanation of Solution
The given equation is:
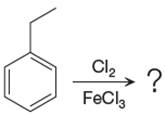
In this aromatic compound, the benzene ring has ethyl group attached. The ethyl group is electron donating inductively and activates the ring at ortho-para positions. Thus it is ortho-para director, on chlorination with
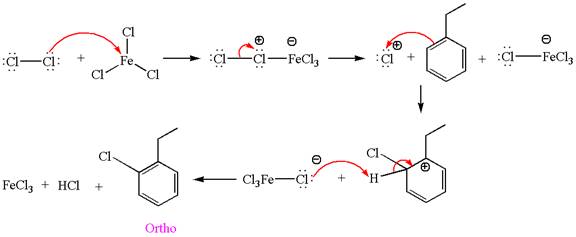
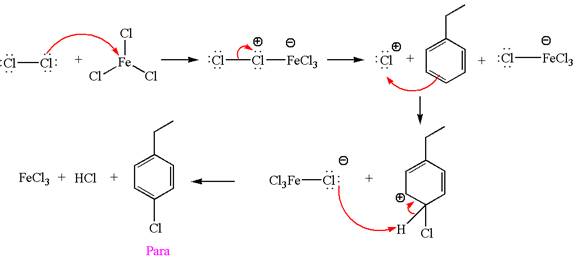
The products with detailed mechanisms are drawn by identifying the substituent at ring is ortho-para director and on chlorination introduced chlorine atom to ring.
(c)
Interpretation:
The products with detailed mechanisms for the formation of each product are to be drawn.
Concept introduction:
The aromatic ring on reaction with acyl chloride in
Answer to Problem 23.34P
The products with detailed mechanisms for the formation of each product are:
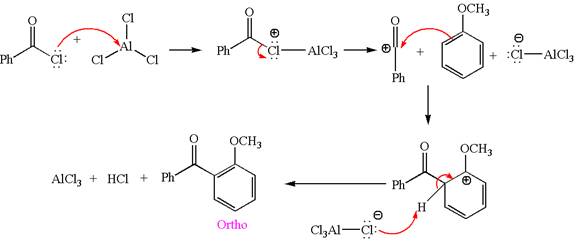
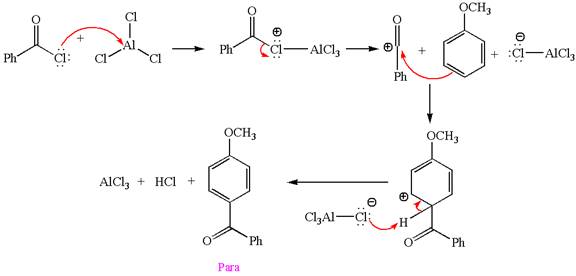
Explanation of Solution
The given equation is:
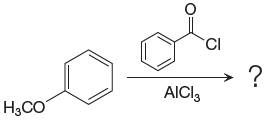
In this aromatic compound, the benzene ring has methoxy group attached. The methoxy group is electron donating and activates the ring at ortho-para positions. Thus it is ortho-para director, on Friedel-Craft acylation in presence of
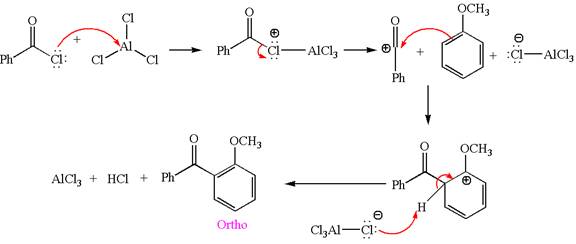
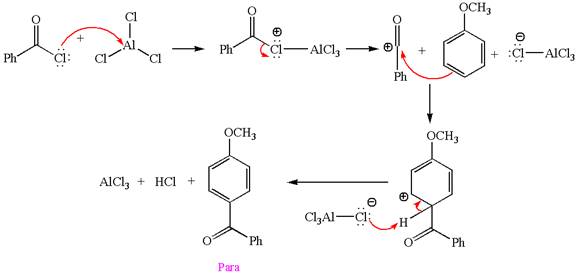
The products with detailed mechanisms are drawn by identifying the substituent at ring is ortho-para director and on F.C. acylation introduced acyl group to ring.
(d)
Interpretation:
The major product with detailed mechanism for the given reaction is to be drawn.
Concept introduction:
The bromination of aromatic ring can be carried out by reacting with
Answer to Problem 23.34P
The major product with detailed mechanism for the given reaction is:
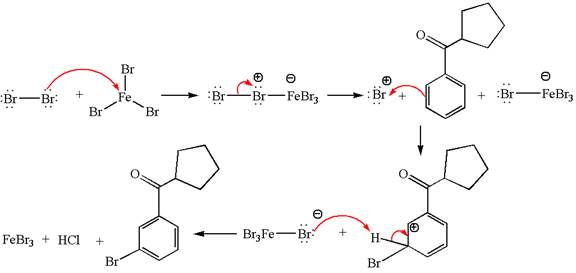
Explanation of Solution
The given equation is:
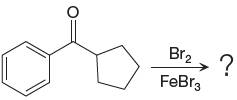
In this aromatic compound, the benzene ring has carbonyl group attached. The carbonyl group is electron withdrawing and deactivates the ring at ortho-para positions. Thus it is meta director, on bromination with

The product with detailed mechanism is drawn by identifying the substituent at ring is meta director and on bromination introduced bromine atom to ring.
(e)
Interpretation:
The products with detailed mechanisms for the formation of each product are to be drawn.
Concept introduction:
The nitration is the electrophilic aromatic substitution reaction. The aromatic ring on reaction with nitric acid or with a mixture of concentrated nitric acid and sulfuric acid undergoes substitution of one of the ring hydrogen by nitro group and forms nitrobenzene, this is called nitration. The electron donating groups activates the aromatic ring and increases the electron density at ortho-para position comparatively than meta position. That means electrophilic aromatic substitution preferably occurs at ortho-para position.
Answer to Problem 23.34P
The products with detailed mechanisms for the formation of each product are:
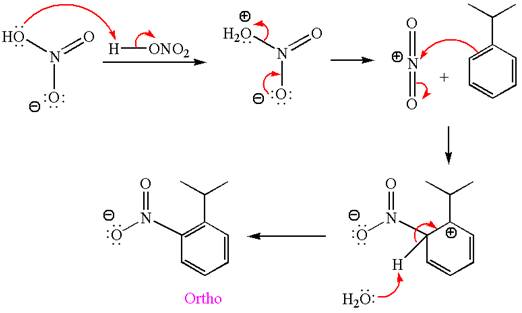
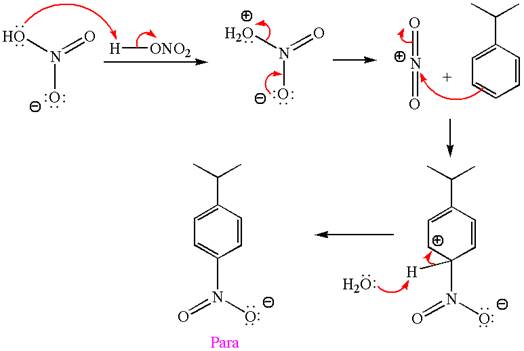
Explanation of Solution
The given equation is:
In this aromatic compound, the benzene ring has methoxy group attached. The methoxy group is electron donating and activates the ring at ortho-para positions. Thus it is ortho-para director, on nitration using
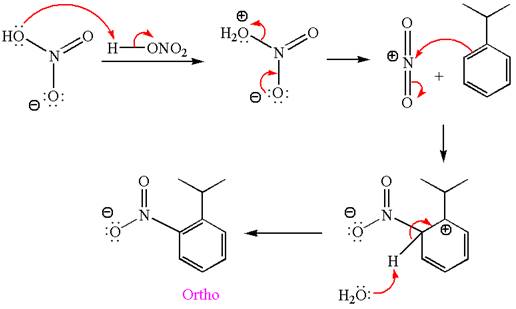
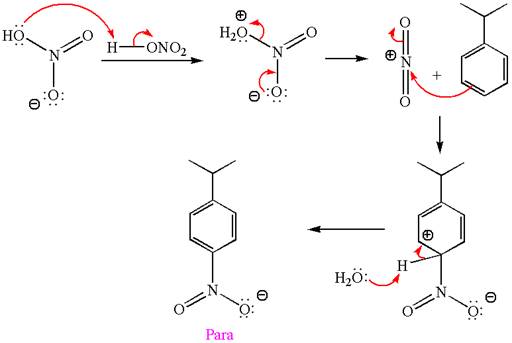
The products with detailed mechanisms are drawn by identifying the substituent at ring is ortho-para director and on nitration introduced nitro group to ring.
Want to see more full solutions like this?
Chapter 23 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- we were assigned to dilute 900ppm in to 18ppm by using only 250ml vol flask. firstly we did calc and convert 900ppm to 0.9 ppm to dilute in 1 liter. to begin the experiment we took 0,225g of kmno4 and dissolved in to 250 vol flask. then further we took 10 ml sample sol and dissolved in to 100 ml vol flask and put it in to a spectrometer and got value of 0.145A . upon further calc we got v2 as 50ml . need to find DF, % error (expval and accptVal), molarity, molality. please write the whole report. thank you The format, tables, introduction, procedure and observation, result, calculations, discussion and conclusionarrow_forwardQ5. Predict the organic product(s) for the following transformations. If no reaction will take place (or the reaction is not synthetically useful), write "N.R.". Determine what type of transition state is present for each reaction (think Hammond Postulate). I Br₂ CH3 F2, light CH3 Heat CH3 F₂ Heat Br2, light 12, light CH3 Cl2, light Noarrow_forwardNonearrow_forward
- In the phase diagram of steel (two components Fe and C), region A is the gamma austenite solid and region B contains the gamma solid and liquid. Indicate the degrees of freedom that the fields A and B have,arrow_forwardFor a condensed binary system in equilibrium at constant pressure, indicate the maximum number of phases that can exist.arrow_forwardPart V. Label ad match the carbons in compounds Jane and Diane w/ the corresponding peak no. in the Spectra (Note: use the given peak no. To label the carbons, other peak no are intentionally omitted) 7 4 2 -0.13 -0.12 -0.11 -0.10 -0.08 8 CI Jane 1 -0.09 5 210 200 190 180 170 160 150 140 130 120 110 100 -8 90 f1 (ppm) 11 8 172.4 172.0 f1 (ppr HO CI NH Diane 7 3 11 80 80 -80 -R 70 60 60 2 5 -8 50 40 8. 170 160 150 140 130 120 110 100 90 -0 80 70 20 f1 (ppm) 15 30 -20 20 -60 60 -0.07 -0.06 -0.05 -0.04 -0.03 -0.02 -0.01 -0.00 -0.01 10 -0.17 16 15 56 16 -0.16 -0.15 -0.14 -0.13 -0.12 -0.11 -0.10 -0.09 -0.08 -0.07 -0.06 -0.05 -0.04 17.8 17.6 17.4 17.2 17.0 f1 (ppm) -0.03 -0.02 550 106 40 30 20 20 -0.01 -0.00 F-0.01 10 0arrow_forward
- n Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forwardPart VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





