
(a)
Interpretation:
The most likely site(s) of electrophilic
Concept introduction:
Electrophilic aromatic substitution reaction rate increases with increase in electron density around the ring. The electron donating groups activates whereas the electron withdrawing groups deactivates the aromatic ring. The electron donating groups increases the electron density at ortho-para position and thus they are ortho-para directing. The electron withdrawing groups decrease electron density at ortho/para and has comparatively greater electron density at meta position, thus they are meta directing.
Answer to Problem 23.53P
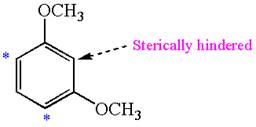
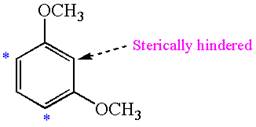
The most likely site(s) electrophilic aromatic substitution in the given molecule is:
Explanation of Solution
The given molecule is:
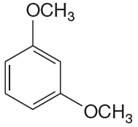
In the given molecule, benzene has two methoxy substituents. These are activating groups, thus ortho-para directors. Each methoxy group activates their two ortho and one para positions. The activated sites are same for both groups, thus overall there are three activated sites. But the position between two methoxy groups is sterically hindered. Thus, the molecules have only two possible sites of electrophilic aromatic substitution which are indicated by an asterisk
The most likely site(s) of electrophilic aromatic substitution are determined by identifying positions activated by the substituents attached.
(b)
Interpretation:
The most likely site(s) of electrophilic aromatic substitution in the given molecule are to be indicated.
Concept introduction:
Electrophilic aromatic substitution reaction rate increases with increase in electron density around the ring. The electron donating groups activate whereas the electron withdrawing groups deactivate the aromatic ring. The electron donating groups increase the electron density at ortho-para position and thus they are ortho-para directing. The electron withdrawing groups decreases electron density at ortho/para and has comparatively greater electron density at meta position, thus they are meta directing.
Answer to Problem 23.53P
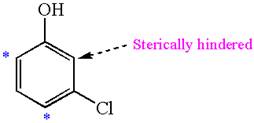
The most likely site(s) of electrophilic aromatic substitution in the given molecule are:
Explanation of Solution
The given molecule is:
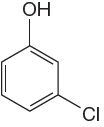
In the given molecule, benzene has one hydroxyl and one chloride substituent. Both these are activating groups, thus ortho-para directors. Each of the susbtituents activates their two ortho and one para positions. The activated sites are same for both groups thus overall there are three activated sites. But the position between these two substituents is sterically hindered. Thus, molecules have only two possible sites of electrophilic aromatic substitution which are indicated by an asterisk
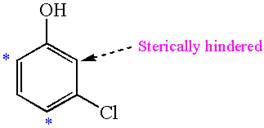
The most likely site(s) of electrophilic aromatic substitution are determined by identifying positions activated by the substituents attached.
(c)
Interpretation:
The most likely site(s) of electrophilic aromatic substitution in the given molecule are to be indicated.
Concept introduction:
The electrophilic aromatic substitution reaction rate increases with increase in electron density around the ring. The electron donating groups activates whereas the electron withdrawing groups deactivates the aromatic ring. The electron donating groups increase the electron density at ortho-para position and thus they are ortho-para directing. The electron withdrawing groups decrease electron density at ortho/para and have comparatively greater electron density at meta position, thus they are meta directing.
Answer to Problem 23.53P
The most likely site(s) of electrophilic aromatic substitution in the given molecule are:
Explanation of Solution
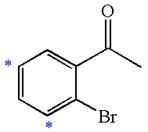
The given molecule is:
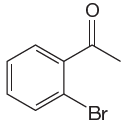
In the given molecule, benzene has one acetyl and one bromide substituent. Acetyl is carbonyl group which is deactivating thus it is meta director. The bromine atom is activating group thus it is ortho/para director. The acetyl group activates its two meta positions and bromine activates its one ortho and one para positions. The meta positions of acetyl group are same as the ortho/para position of bromine atom. Thus the molecules have only two possible sites of electrophilic aromatic substitution which are indicated by an asterisk
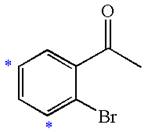
The most likely site(s) of electrophilic aromatic substitution are determined by identifying positions activated by the substituents attached.
(d)
Interpretation:
The most likely site(s) of electrophilic aromatic substitution in the given molecule are to be indicated.
Concept introduction:
The electrophilic aromatic substitution reaction rate increases with increase in electron density around the ring. The electron donating groups activates whereas the electron withdrawing groups deactivates the aromatic ring. The electron donating groups increases the electron density at ortho-para position and thus they are ortho-para directing. The electron withdrawing groups decreases electron density at ortho/para and has comparatively greater electron density at meta position, thus they are meta directing.
Answer to Problem 23.53P
The most likely site(s) of electrophilic aromatic substitution in the given molecule are:
Explanation of Solution
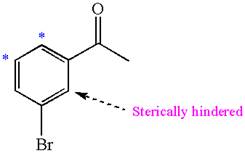
The given molecule is:
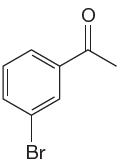
In the given molecule, benzene has one acetyl and one bromide substituent. Acetyl is carbonyl group which is deactivating thus it is meta director. The bromine atom is activating group thus it is ortho/para director.
The acetyl group activates its one meta positions and bromine activates two ortho and one para positions. The activating sites of both groups are different, thus overall there are three activated sites. But the position between these two substituents is sterically hindered. Thus, molecules have only two possible sites of electrophilic aromatic substitution which are indicated by an asterisk

The most likely site(s) of electrophilic aromatic substitution are determined by identifying positions activated by the substituents attached.
(e)
Interpretation:
The most likely site(s) of electrophilic aromatic substitution in the given molecule are to be indicated.
Concept introduction:
The electrophilic aromatic substitution reaction rate increases with increase in electron density around the ring. The electron donating groups activates whereas the electron withdrawing groups deactivates the aromatic ring. The electron donating groups increases the electron density at ortho-para position and thus they are ortho-para directing. The electron withdrawing groups decreases electron density at ortho/para and has comparatively greater electron density at meta position, thus they are meta directing.
Answer to Problem 23.53P
The most likely site(s) of electrophilic aromatic substitution in the given molecule are:
Explanation of Solution
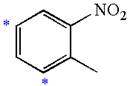
The given molecule is:
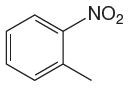
In the given molecule, benzene has one nitro and one methyl substituent. Nitro group is deactivating thus it is meta director. The methyl group is activating group thus it is ortho/para director. The nitro group activates its two meta positions and methyl group activates its one ortho and one para positions. The meta positions of nitro group are same as the ortho/para positions of methyl groups. Thus, the molecules have only two possible sites of electrophilic aromatic substitution which are indicated by an asterisk

The most likely site(s) of electrophilic aromatic substitution are determined by identifying positions activated by the substituents attached.
(f)
Interpretation:
The most likely site(s) of electrophilic aromatic substitution in the given molecule are to be indicated.
Concept introduction:
The electrophilic aromatic substitution reaction rate increases with increase in electron density around the ring. The electron donating groups activates whereas the electron withdrawing groups deactivates the aromatic ring. The electron donating groups increases the electron density at ortho-para position and thus they are ortho-para directing. The electron withdrawing groups decreases electron density at ortho/para and has comparatively greater electron density at meta position, thus they are meta directing.
Answer to Problem 23.53P
The most likely site(s) of electrophilic aromatic substitution in the given molecule are:
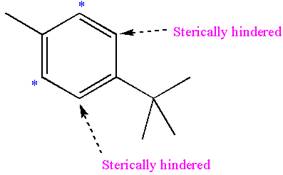
Explanation of Solution
The given molecule is:
In the given molecule, benzene has one methyl and one

The most likely site(s) of electrophilic aromatic substitution are determined by identifying positions activated by the substituents attached.
(g)
Interpretation:
The most likely site(s) of electrophilic aromatic substitution in the given molecule are to be indicated.
Concept introduction:
The electrophilic aromatic substitution reaction rate increases with increase in electron density around the ring. The electron donating groups activates whereas the electron withdrawing groups deactivates the aromatic ring. The electron donating groups increases the electron density at ortho-para position and thus they are ortho-para directing. The electron withdrawing groups decreases electron density at ortho/para and has comparatively greater electron density at meta position, thus they are meta directing.
Answer to Problem 23.53P
The most likely site(s) of electrophilic aromatic substitution in the given molecule are:
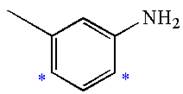
Explanation of Solution
The given molecule is:
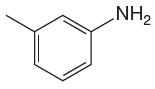
In the given molecule, benzene has one methyl and one amino substituent. They both are activating groups, thus they are ortho/para positions. Each of the susbtituents activates their two ortho and one para positions. The some activated sites are not same for both groups so, overall there are three activated sites. But the position between two groups is sterically hindered. Thus, molecules have only two possible sites of electrophilic aromatic substitution which are indicated by an asterisk

The most likely site(s) of electrophilic aromatic substitution are determined by identifying positions activated by the substituents attached.
Want to see more full solutions like this?
Chapter 23 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- If we have the equation 3 ln0 = 2t. What is the value of t?arrow_forward1 N2H4 (l) + 3 O2(g) > 2 NO2 (g) + 2 H2O (g) If 75.0 kg of hydrazine are reacted with 75.0 kg of oxygen, which is the limiting reactant?arrow_forwardPQ-10. What is the major product of this reaction? (A) (C) 930 Me HO O=S=O O-8-CF, C 어 Me H+ OH 270 O 0-5-0 O=S=O O-S-CF CF3 2arrow_forward
- Predict the major organic product(s) of the following reactions. Include stereochemistry when necessary. Write NR if no reaction, try to explain.arrow_forwardQ2: Explain why epoxides that react in an SN1 manner will not show any stereochemical inversion in the product. Q3: Rationalize why Alcohol B will react under the indicated reaction conditions, but Alcohol A will not. A ☑ OH B OH PBr3 R-Brarrow_forwardQ1: Predict the major organic product(s) of the following reactions. Include stereochemistry when necessary. Write NR if no reaction, try to explain. 1.) LDA, THF 2.) СОН CI OH H2SO4, heat OH m...... OH 1.) PCC, CH2Cl2 2.) CH3CH2MgBr, THF 3.) H3O+ 4.) TsCl, pyr 5.) tBuOK, tBuOH 1.) SOCI 2, CHCI 3 2.) CH3CH2ONA, DMF OH 1.) HBr 2.) Mg, THF 3.) H₂CO, THE 4.) H3O+ OH NaH, THFarrow_forward
- Problem 6-29 Identify the functional groups in the following molecules, and show the polarity of each: (a) CH3CH2C=N CH, CH, COCH (c) CH3CCH2COCH3 NH2 (e) OCH3 (b) (d) O Problem 6-30 Identify the following reactions as additions, eliminations, substitutions, or rearrangements: (a) CH3CH2Br + NaCN CH3CH2CN ( + NaBr) Acid -OH (+ H2O) catalyst (b) + (c) Heat NO2 Light + 02N-NO2 (+ HNO2) (d)arrow_forwardPredict the organic product of Y that is formed in the reaction below, and draw the skeletal ("line") structures of the missing organic product. Please include all steps & drawings & explanations.arrow_forwardPlease choose the best reagents to complete the following reactionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





