
(a)
Interpretation:
The isomer from the given pair that will undergo electrophilic
Concept introduction:
The electron donating groups activate whereas the electron withdrawing groups deactivate the aromatic ring. The electron donating groups are ortho-para directing, which means electrophilic aromatic substitution preferably occurs at the ortho-para position.
Answer to Problem 23.46P
The
Explanation of Solution
The given pair is
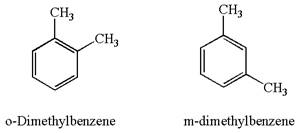
In both structures, benzene has two methyl substituents, which are activating groups. They activate the ring at ortho-para direction. As the position of methyl groups is different in these structures, their activating position on the ring would be different. In both the structures, each methyl activates the ring at two positions, as shown below:
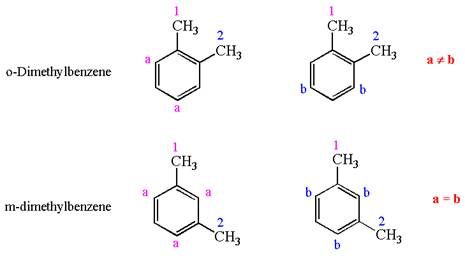
The activating positions of first methyl are indicated by letter
It is determined that
(b)
Interpretation:
The isomer from the given pair that will undergo electrophilic aromatic substitution faster is to be determined.
Concept introduction:
The electron donating groups activate whereas the electron withdrawing groups deactivate the aromatic ring. The electron donating groups are ortho-para directing, which means electrophilic aromatic substitution preferably occurs at the ortho-para position.
Answer to Problem 23.46P
The
Explanation of Solution
The given pair is
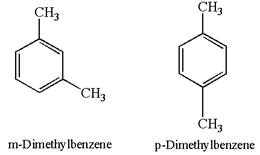
In both structures, the benzene has two methyl substituents, which are activating groups. They activate the ring at ortho-para direction. As the position of methyl groups is different in these structures, their activating position on ring would be different. In both the structures, each methyl activates the ring at two positions, as shown below:
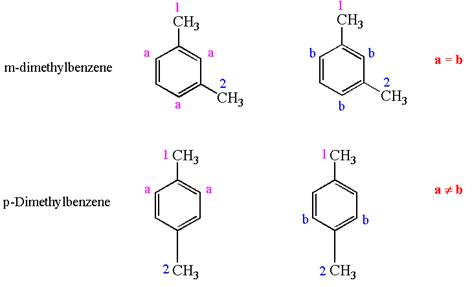
The activating positions of first methyl are indicated by letter
It is determined that
(c)
Interpretation:
The isomer from the given pair that will undergo electrophilic aromatic substitution faster is to be determined.
Concept introduction:
The electron donating groups activate whereas the electron withdrawing groups deactivate the aromatic ring. The electron donating groups are ortho-para directing, which means electrophilic aromatic substitution preferably occurs at the ortho-para position.
Answer to Problem 23.46P
The
Explanation of Solution
The given pair is
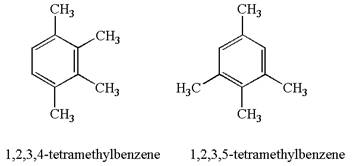
In both structures, the benzene has four methyl substituents, which are activating groups. They activate the ring at ortho-para direction. As the position of methyl groups is different in these structures, their activating position on ring would be different. In both the structures, each methyl activates the ring at two positions, as shown below:
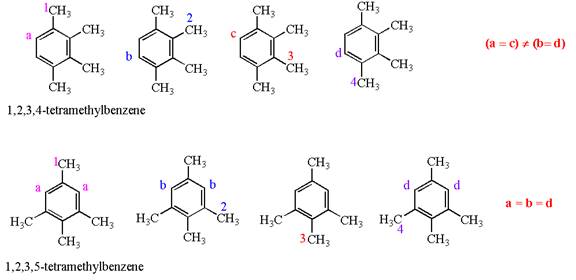
The activating positions of first methyl are indicated by letter
In case of
The methyl groups in
It is determined that
Want to see more full solutions like this?
Chapter 23 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- What is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forwardΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forward
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





