
Concept explainers
Interpretation:
The number of stereoisomers possible for
Concept Introduction:
The pair of diastereomers that shows different configuration at the chirality center are called epimers.
Monosaccharide, having its highest chirality center same as that of
The pair of isomers of a molecule that are mirror images of one another are called enantiomers.
The molecules or compounds which are non-superimposable or not identical with its mirror image are known as chiral molecules.
The pair of two mirror images which are non-identical are known as enantiomers and these are optically active.
The objects or molecules which are superimposable with their mirror images are achiral objects or molecules and these objects have a centre of symmetry or plane of symmetry.
The achiral compounds in which plane of symmetry is present internally and consists of chiral centres are known as meso compounds but they are optically inactive.
The stereoisomers which are non-superimposable on each other and not mirror images of each other are known as diastereomers.
Chiral molecules are capable of rotating plane polarized light
The molecules which are superimposable or identical with their mirror images are known as achiral molecules, and achiral molecules are not capable of rotating the plane-polarised light.
Plane of symmetry is the plane that bisects the molecule in two equal halves, such that they are mirror images of each other.
Compounds having plane of symmetry are usually achiral as they do not have different atoms around the central carbon atom.
Threo and erythro are commonly used to distinguish diastereomers.
In fischer projection, the erythro isomer has two identical substituents on the same side.
In fischer projection, the threo isomer has two identical substituents on the opposite side.
In zig-zag formula, the erythro isomer has two identical substituents on different sides of the plane.
The names are derived from the diastereomeric aldoses erythrose and threose.
In double bond or cyclic compounds, if two same functional groups are present on the same side of the double bond or cyclic compound, the given compound can be labeled as cis.
If the two functional groups are present on the different sides of the double bond or cyclic compound, the given compound can be labeled as Trans.
Cis-trans isomerism exists in the compounds in which similar groups are present on the adjacent carbon atoms.
Answer to Problem 1PP
Solution:
a)
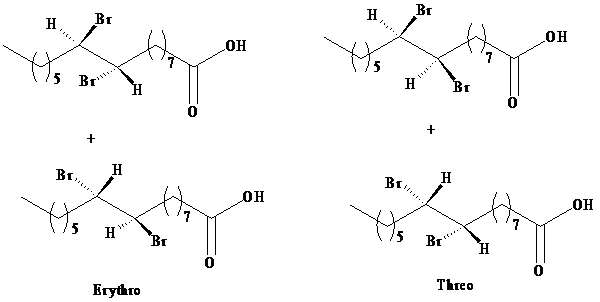
Two sets of enantiomers of erythrose and threose are given as:
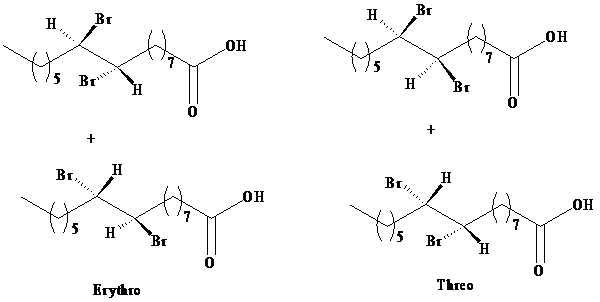
b)
The three-dimensional structures of the given compound are as:
Explanation of Solution
a) The number of stereoisomers are possible for
The product obtained from the reaction of bromine with palmitoleic acid is a mixture of erythrose and threose. The designations erythro and threo come from the names of the sugars called erythrose and threose. Both
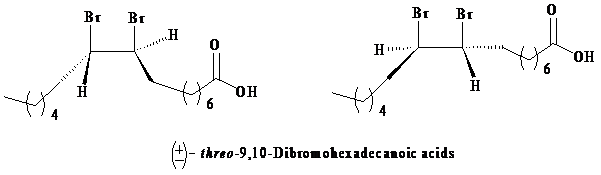
b) The three-dimensional structures for the
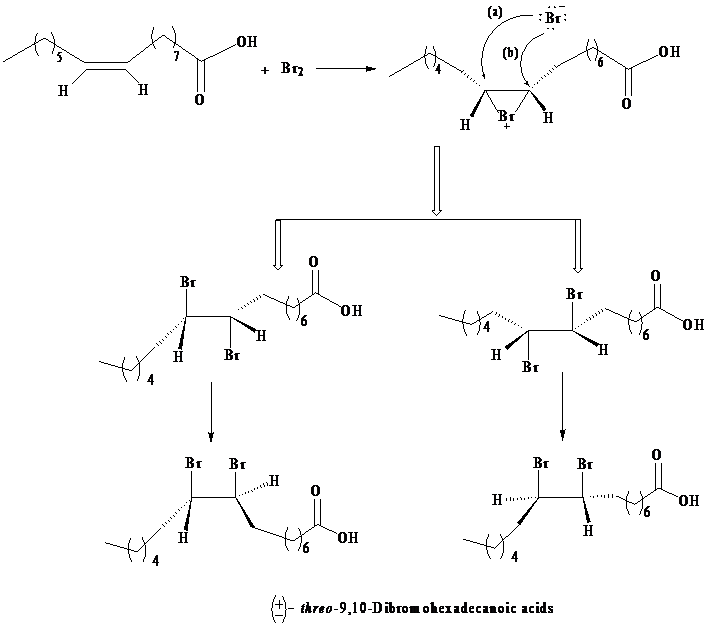
As per the information given in the question, the addition of bromine is an anti-addition to the double bond, that is, it takes place through bromonium ion as an intermediate. Formation of a bromonium ion at the other end of the palmitoleic acid gives a result, such that only threo enantiomers are produced due to the racemic modification.
The three-dimensional structures of the
Want to see more full solutions like this?
Chapter 23 Solutions
ORGANIC CHEMISTRY-WILEYPLUS ACCESS PKG.
- Calculate the ionization energy of He+ and Li²+ ions in their ground states. Thannnxxxxx sirrr Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdvaarrow_forwardPlleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AIarrow_forwardCalculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forward
- 4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardIII O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward
- 3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forwardWhat is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

