
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.3, Problem 12P
Using the pKa values in Section 2.3, rank the following species in order from strongest base to weakest base:
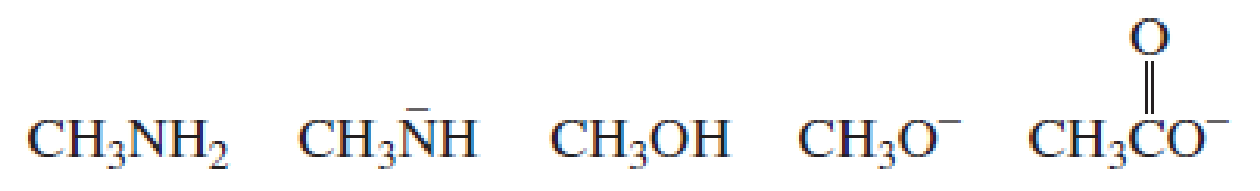
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Describe the mesomeric or resonance effect and differentiate between types +E or +M and -R or -M.
I need help with the following two problems, understanding them in a simple manner. Can you please draw them out for me with a detailed explanation so that I can better comprehend? I'm a visual person, so I definitely need that. Thank you very much!
Problem 54, could you please explain it in detail? Thank you! Step by step, I'm really confused, so please don't make it overly complex. My question is to visually draw it out and demonstrate it to me; I'm confused about that problem, please (not just in words) but demonstrate it to me in all due essence (visually) with descriptions.
Chapter 2 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 2.1 - Which of the following are not acids? CH3COOH CO2...Ch. 2.1 - Draw the products of the acidbase reaction when a....Ch. 2.1 - a.What is the conjugate acid of each of the...Ch. 2.2 - a. Which is a stronger acid, one with a pKa of 5.2...Ch. 2.2 - Prob. 5PCh. 2.2 - Antacids are compounds that neutralize stomach...Ch. 2.2 - Are the following body fluids acidic or basic? a....Ch. 2.3 - Draw the conjugate acid of each of the following:...Ch. 2.3 - a. Write an equation showing CH3OH reacting as an...Ch. 2.3 - Prob. 10P
Ch. 2.3 - a. Which is a stronger base, CH3COO or HCOO? (The...Ch. 2.3 - Using the pKa values in Section 2.3, rank the...Ch. 2.4 - Prob. 13PCh. 2.5 - Prob. 14PCh. 2.5 - Ethyne has a pKa value of 25, water has a pKa...Ch. 2.5 - Which of the following bases can remove a proton...Ch. 2.6 - List the ions (CH3, NH2, HO, and F) in order from...Ch. 2.6 - List the carbanions shown in the margin in order...Ch. 2.6 - Which is a stronger acid?Ch. 2.6 - a. Draw the products of the following reactions: A...Ch. 2.6 - List the halide ions (F, Cl, Br, and I) in order...Ch. 2.6 - a. Which is more electronegative, oxygen or...Ch. 2.6 - Which is a stronger acid? a. HCl or HBr b....Ch. 2.6 - a. Which of the halide ions (F, Cl, Br, and I) is...Ch. 2.6 - Which is a stronger base? a. H2O or HO b. H2O or...Ch. 2.7 - Which is a stronger acid? a. CH3OCH2CH2OH or...Ch. 2.7 - Which is a stronger base?Ch. 2.8 - Fosamax has six acidic groups. The structure of...Ch. 2.8 - Which is a stronger acid? Why?Ch. 2.10 - For each of the following compounds (shown in...Ch. 2.10 - Prob. 33PCh. 2.11 - Write the equation that shows how a buffer made by...Ch. 2.11 - What products are formed when each of the...Ch. 2 - a. List the following alcohols in order from...Ch. 2 - Which is a stronger base? a. HS or HO b. CH3O or...Ch. 2 - Prob. 40PCh. 2 - a. List the following carboxylic acids in order...Ch. 2 - For the following compound, a. draw its conjugate...Ch. 2 - List the following compounds in order from...Ch. 2 - For each of the following compounds, draw the form...Ch. 2 - Give the products of the following acidbase...Ch. 2 - Prob. 46PCh. 2 - For each compound, indicate the atom that is most...Ch. 2 - Tenormin, a member of the group of drugs known as...Ch. 2 - From which acids can HO remove a proton in a...Ch. 2 - Prob. 50PCh. 2 - Which is a stronger acid? a. CH29CHCOOH or...Ch. 2 - Prob. 52PCh. 2 - Prob. 53PCh. 2 - How could you separate a mixture of the following...Ch. 2 - Prob. 1PCh. 2 - Prob. 2PCh. 2 - Draw the products of the following acidbase...Ch. 2 - Prob. 4PCh. 2 - Prob. 5PCh. 2 - Prob. 6PCh. 2 - Prob. 7PCh. 2 - Prob. 8PCh. 2 - Prob. 9PCh. 2 - Prob. 10PCh. 2 - Prob. 11PCh. 2 - Prob. 12PCh. 2 - Prob. 13PCh. 2 - Prob. 14P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- So, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forwardDraw a mental model for calcium chloride mixed with sodium phosphatearrow_forward
- here is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forwardDrawing of 3-fluro-2methylphenolarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY