
a)
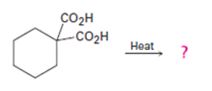
Interpretation:
The products of the reaction shown are to be given.
Concept introduction:
Compounds having two carboxyl groups attached to a carbon readily undergo decarboxylation, when heated, to yield monocarboxylic acids.
To give:
The products of the reaction shown.
b)
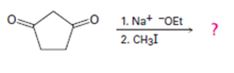
Interpretation:
The product of the reaction shown is to be given.
Concept introduction:
In the first reaction the base abstracts a proton to yield an enolate ion. In the second reaction alkylation takes place.
To give:
The product of the reaction shown.
c)
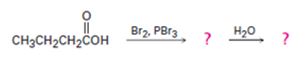
Interpretation:
The products of the reaction shown are to be given.
Concept introduction:
When carboxylic acids are treated with Br2 and PBr3, bromination occurs at the carbon α- to the carboxyl group and the acid group also is converted into an acyl bromide. When treated with water the acyl bromide gets hydrolyzed to yield the free acid.
To give:
The products of the reaction shown.
d)
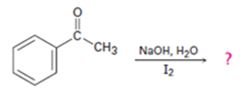
Interpretation:
The products of the reaction shown are to be given.
Concept introduction:
Methyl
To give:
The products of the reaction shown.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- Please explain how to calculate the pH.arrow_forwardI'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forward
- (a) 21.8 Name the following compounds. & (b) Br (e) O₂N. (h) H (c) Br (d) NH2 ☑N Br H ہیں Ph (g) OMe бл .0-0.e 21.9 Draw a structural formula for each compound. (a) 2,3-Dinitrotoluene (c) Diphenylmethanol (e) p-Nitroaniline (b) 3-Propylanisole (d) m-Propylphenol (f) Pentabromobenzenearrow_forwardIs this the major product of this reaction?arrow_forwardPlease helparrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

