
ORG CHEM CONNECT CARD
6th Edition
ISBN: 9781264860746
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22.8, Problem 23P
Which of the following compounds can serve as Michael acceptors?
a. 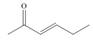 b.
b. 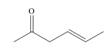 c.
c. 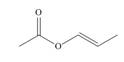 d.
d. 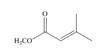
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculate the atomic packing factor of diamond knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are, respectively, 0.038 and 0.117 nm.
A pdf file of your hand drawn, stepwise mechanisms for the reactions.
For each reaction in the assignment, you must write each mechanism
three times (there are 10 reactions, so 30 mechanisms). (A) do the work
on a tablet and save as a pdf., it is expected to write each mechanism
out and NOT copy and paste the mechanism after writing it just once.
Everything should be drawn out stepwise and every bond that is formed
and broken in the process of the reaction, and is expected to see all
relevant lone pair electrons and curved arrows.
Aldol:
NaOH
HO
H
Δ
NaOH
Δ
None
Chapter 22 Solutions
ORG CHEM CONNECT CARD
Ch. 22.1 - Prob. 1PCh. 22.1 - Prob. 2PCh. 22.1 - Problem 24.3
What unsaturated carbonyl compound is...Ch. 22.1 - Prob. 4PCh. 22.1 - Prob. 5PCh. 22.2 - Prob. 6PCh. 22.2 - Problem 24.7
Draw the products formed in each...Ch. 22.4 - Prob. 15PCh. 22.4 - Prob. 16PCh. 22.5 - Problem 24.16
What ester is formed when each...
Ch. 22.6 - Prob. 18PCh. 22.6 - Prob. 19PCh. 22.6 -
Draw the products of each reaction.
a. b.
Ch. 22.6 - Problem 24.20
Two steps in a synthesis of the...Ch. 22.7 - Prob. 22PCh. 22.8 - Problem 24.22
Which of the following compounds can...Ch. 22.8 - Prob. 24PCh. 22.8 - Problem 24.24
What starting materials are needed...Ch. 22 - Prob. 29PCh. 22 - 24.29 What steps are needed to convert A to B?
Ch. 22 - Prob. 31PCh. 22 - 24.31 Draw the product formed in each directed...Ch. 22 - Prob. 33PCh. 22 - 24.33 What starting materials are needed to...Ch. 22 - Prob. 35PCh. 22 - Prob. 36PCh. 22 - 24.36 Identify the structures of C and D in the...Ch. 22 - Prob. 38PCh. 22 - Prob. 39PCh. 22 - 24.39 Draw the product formed from a Claisen...Ch. 22 - Prob. 41PCh. 22 - 24.41 Even though B contains three ester groups, a...Ch. 22 - Prob. 43PCh. 22 - Prob. 44PCh. 22 - 24.44 Vetivone is isolated from vetiver, a...Ch. 22 - Draw the product of each Robinson annulation from...Ch. 22 - Prob. 50PCh. 22 - Prob. 51PCh. 22 - 24.52 Draw a stepwise mechanism for the following...Ch. 22 - Prob. 53PCh. 22 - Prob. 54PCh. 22 - Prob. 55PCh. 22 - Prob. 56PCh. 22 - Prob. 57PCh. 22 - Prob. 58PCh. 22 - Prob. 59PCh. 22 - 24.62 Devise a synthesis of each compound from ,...Ch. 22 - Prob. 63P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw structures corresponding to the following names and give IUPAC names for the following compounds: (8 Point) a) b) c) CH3 CH2CH3 CH3CHCH2CH2CH CH3 C=C H3C H H2C=C=CHCH3 d) CI e) (3E,5Z)-2,6-Dimethyl-1,3,5,7-octatetraene f) (Z)-4-bromo-3-methyl-3-penten-1-yne g) cis-1-Bromo-2-ethylcyclopentane h) (5R)-4,4,5-trichloro-3,3-dimethyldecanearrow_forwardNonearrow_forwardReview: Design a total total synthesis synthesis of the following compound using methyloxacyclopropane and any other necessary reagents.arrow_forward
- Nonearrow_forwardDraw a Newman projection from carbon 3 to carbon 2 in the highest energy conformation for the following molecule. What is this conformation called? What kind of strain is present? Brarrow_forwardWhich of the following dienophiles is most reactive in a Diels-Alder reaction: Please explain why the correct answer to this question is option 5. Please provide a detailed explanation.arrow_forward
- Which of the following would you expect to be aromatic? Please provide a detailed explanation.arrow_forwardDraw the enantiomer and diastereomers of the following molecule. Label each type of stereoisomers. Label each chiral center as R or S. HOarrow_forwardWhich diene and dienophile would you choose to synthesize the following compound? Please provide a detailed explanation. Please include a drawing showing the mechanism of the synthesis. Please also explain why it is the correct diene and dienophile.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY