
Concept explainers
(a)
Interpretation:
Whether the statement “The most common primary protein structures are the
Concept introduction:
Proteins are the
Answer to Problem 60E
The statement “The most common primary protein structures are the
Explanation of Solution
Primary structure of a protein is straight chain polymer of amino acids linked by the amide linkages. The
The statement “The most common primary protein structures are the
(b)
Interpretation:
Whether the statement “Sulfur-sulfur bonds called disulfide linkages are important in the tertiary protein structure of many proteins” is true or false is to be stated.
Concept introduction:
Proteins are the polymers of amino acids also known as polypeptides. They are formed by the peptide bonding between two acids formed by the elimination of the water molecule between the carboxylic acid and amine group leading to the formation of the amide group. Various structure or forms of the proteins are found in the body.
Answer to Problem 60E
The statement “Sulfur-sulfur bonds called disulfide linkages are important in the tertiary protein structure of many proteins” is a true statement.
Explanation of Solution
The tertiary structure of the proteins is curling of the secondary protein structure on itself. The stability of the tertiary structure of many tertiary proteins is enhanced by the sulfur-sulfur linkages between the sulfur-containing amino acids, for example, Cysteine.
Therefore, the statement “Sulfur-sulfur bonds called disulfide linkages are important in the tertiary protein structure of many proteins” is true.
The statement “Sulfur-sulfur bonds called disulfide linkages are important in the tertiary protein structure of many proteins” is a true statement.
(c)
Interpretation:
Whether the statement “An enzyme substrate is the product of an enzyme-catalyzed reaction” is true or false is to be stated.
Concept introduction:
Enzymes are the biochemical catalysts present in the human body or livings organism to catalyst their biochemical process. Enzymes fasten up the
Answer to Problem 60E
The statement “An enzyme substrate is the product of an enzyme-catalyzed reaction” is a false statement.
Explanation of Solution
The substrate in an enzyme-catalyzed reaction is the reactant reacting with the enzyme to give a back product. Substrate attaches to the active site of the enzyme for the catalysis. Therefore, the statement “An enzyme substrate is the product of an enzyme-catalyzed reaction” is a false statement.
The statement “An enzyme substrate is the product of an enzyme-catalyzed reaction” is a false statement.
(d)
Interpretation:
Whether the statement “The catalytic properties of an enzyme exist at its active site” is true or false is to be stated.
Concept introduction:
Enzymes are the biochemical catalysts present in the human body or livings organism to catalyst their biochemical process. Enzymes fasten up the rate of reaction by a very large factor. Substrate comes to the active site of the enzyme for the catalysis.
Answer to Problem 60E
The statement “The catalytic properties of an enzyme exist at its active site” is a true statement.
Explanation of Solution
The substrate in an enzyme-catalyzed reaction is the reactant reacting with the enzyme to give a back product. Substrate attaches to the active site of the enzyme for the catalysis. Therefore, the statement “The catalytic properties of an enzyme exist at its active site” is true.
The statement “The catalytic properties of an enzyme exist at its active site” is a true statement.
(e)
Interpretation:
Whether the statement “Carbohydrates are the hydrates of carbon; that is, compounds that consist of carbon and water molecules” is true or false is to be stated.
Concept introduction:
Sugar molecules are examples of carbohydrates. Sugars are the
Answer to Problem 60E
The statement “Carbohydrates are the hydrates of carbon; that is, compounds that consist of carbon and water molecules” is a false statement.
Explanation of Solution
Carbohydrates are thought to be “hydrates of carbon” as suggested by the name but they are classified as the aldehydes or ketones containing two or more hydroxide
The statement “Carbohydrates are the hydrates of carbon; that is, compounds that consist of carbon and water molecules” is a false statement.
(f)
Interpretation:
Whether the statement “Glucose is also called blood sugar” is true or false is to be stated.
Concept introduction:
Glucose is a sugar molecule also known as carbohydrates. It is a six carbon aldose sugar which means it contains aldehyde and hydroxide groups. Glucose has two forms
Answer to Problem 60E
The statement “Glucose is also called blood sugar” is a true statement.
Explanation of Solution
Glucose is a sugar molecule also known as carbohydrates. It is a six carbon aldose sugar which means it contains aldehyde and hydroxide groups. Glucose has two forms
The statement “Glucose is also called blood sugar” is a true statement.
(g)
Interpretation:
Whether the statement “Sucrose is a glucose-fructose combination” is true or false is to be stated.
Concept introduction:
Carbohydrates are classified as the compounds that are aldehydes or ketone containing two or more
Answer to Problem 60E
The statement “Sucrose is a glucose-fructose combination” is a true statement.
Explanation of Solution
The disaccharide is the combination of two monosaccharides may be identical or different. Sucrose is one of the most famous examples of disaccharides. It is the combination of two monosaccharide units glucose and fructose. Therefore, the statement “Sucrose is a glucose-fructose combination” is true.
The statement “Sucrose is a glucose-fructose combination” is a true statement.
(h)
Interpretation:
Whether the statement “Fats and oils are distinguished by their
Concept introduction:
Lipids are the water-insoluble compounds found in the living organisms. Lipids are parted into three classes (1) Fats, oils and phospholipids, (2) waxes, and (3) steroids.
All these compounds are insoluble in polar solvents like water because of the large presence of hydrophobic groups in them.
Answer to Problem 60E
The statement “Fats and oils are distinguished by their state of matter at room temperature” is a true statement.
Explanation of Solution
Fats and oils are the triesters of long-chain carboxylic acids known as fatty acids. The ester is formed from simple alcohol glycerol. The fats and oils are also referred to as triacylglycerols. The fats at room temperature are solid while the oils are liquid at room temperature. Therefore, the statement “Fats and oils are distinguished by their state of matter at room temperature” is true.
The statement “Fats and oils are distinguished by their state of matter at room temperature” is a true statement.
(i)
Interpretation:
Whether the statement “Steroids have three cyclopentane rings and one cyclohexane ring fused” is true or false is to be stated.
Concept introduction:
Lipids are the water-insoluble compounds found in the living organisms. Lipids are parted into three classes (1) Fats, oils and phospholipids, (2) waxes, and (3) steroids.
All these compounds are insoluble in polar solvents like water because of the large presence of hydrophobic groups in them.
Answer to Problem 60E
The statement “Steroids have three cyclopentane rings and one cyclohexane ring fused” is a false statement.
Explanation of Solution
Steroids contain a four ring fused system. Three are six carbon and one is five carbon ring. Six carbon ring is known as cyclohexane and five carbon ring is known as cyclopentane. Therefore, the statement “Steroids have three cyclopentane rings and one cyclohexane ring fused” is false.
The structure of one of the steroids cholesterol is shown below.
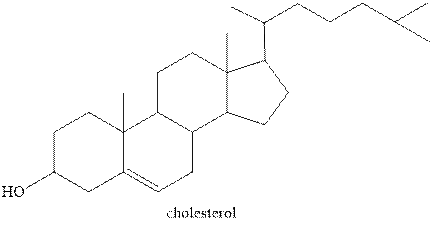
Figure 1
The statement “Steroids have three cyclopentane rings and one cyclohexane ring fused” is a false statement.
(j)
Interpretation:
Whether the statement “Nucleic acid monomers are called nucleotides” is true or false is to be stated.
Concept introduction:
Nucleotides are the monomers of
Answer to Problem 60E
The statement “Nucleic acid monomers are called nucleotides” is a true statement.
Explanation of Solution
Nucleic acids are the polymer of nucleotides. Nucleotides are phosphate and base-sugar combination. Nucleotides contain two types of sugars in them one is ribose sugar and other is deoxyribose sugar.
Therefore, the statement “Nucleic acid monomers are called nucleotides” is true.
The statement “Nucleic acid monomers are called nucleotides” is a true statement.
(k)
Interpretation:
Whether the statement “DNA is a nucleotide (phosphate-sugar-base) polymer” is true or false is to be stated.
Concept introduction:
Nucleotides are the monomers of nucleic acids. DNA and RNA are examples of nucleotides. Nucleotides consist of three parts (a) cyclic nitrogenous base (b) a sugar (ribose or deoxyribose sugar) (c) phosphate group.
Answer to Problem 60E
The statement “DNA is a nucleotide (phosphate-sugar-base) polymer” is a true statement.
Explanation of Solution
Nucleic acids are nucleotide polymers. Nucleotides are a combination of phosphate and base-sugar. DNA is one of the examples of nucleic acids. DNA contains nucleotides having deoxyribose sugar and specific bases (adenine, guanine, thymine, and cytosine).
Therefore, the statement “DNA is a nucleotide (phosphate-sugar-base) polymer” is true.
The statement “DNA is a nucleotide (phosphate-sugar-base) polymer” is a true statement.
Want to see more full solutions like this?
Chapter 22 Solutions
Introductory Chemistry: An Active Learning Approach
- > Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardName the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forward
- How to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forwardPredict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forward
- Predict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardFor the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forwardUsing the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forward
- Using arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forwarddraw out the following structures plesearrow_forwardDraw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





