
Concept explainers
(a)
Interpretation:
The terms secondary protein structure and tertiary protein structure are to be distinguished in the scientific terms.
Concept introduction:
Proteins are the
Answer to Problem 59E
The secondary protein structure is found in the form of
Explanation of Solution
The secondary structure of the proteins is found in the form of

Figure 1
The terms secondary protein structure and tertiary protein structure are distinguished rightfully above.
(b)
Interpretation:
The terms
Concept introduction:
Proteins are the polymers of amino acids also known as polypeptides. They are formed by the peptide bonding between two acids formed by the elimination of the water molecule between the carboxylic acid and amine group leading to the formation of the amide group. Various structure or forms of the proteins are found in the body.
Answer to Problem 59E
The protein’s secondary structure is one of the protein structures. It is discovered in two forms,
Explanation of Solution
Secondary structure of the protein is one of the structures of the proteins. It is found in two forms
The structure of
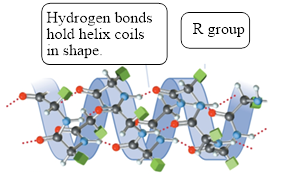
Figure 2
The structure of
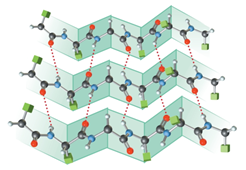
Figure 3
The terms
(c)
Interpretation:
The terms reversible enzyme inhibitors and irreversible enzyme inhibitors are to be distinguished in the scientific terms.
Concept introduction:
Enzymes are the biochemical catalysts present in the human body or livings organism to catalyst their biochemical process. Enzymes fasten up the
Inhibitors are the compounds that stops the working of the enzyme as a catalyst. Inhibitors are of two reversible enzyme inhibitors and irreversible enzyme inhibitors.
Answer to Problem 59E
The enzyme includes an active site where the molecule of the substrate arrives and binds to the catalysis with the enzyme. The active site has the supplementary components displaying attraction forces to bind the molecule of the substrate to it. In the induced fit model, the bonding of the substrate with an enzyme was assumed to be reversible. That is, after the job is finished, it will leave the active site. Reversible enzyme inhibitors are molecules comparable to substrate molecules with the same type of atom or groups on the outside complementing the forces of attraction to occur at the active site. In this manner, the inhibitor blocks the active site accessible for the substrate and inhibits the biochemical reaction. Sometimes it has been discovered that the substrate stays attached to the active site which is its connection is irreversible. The compounds performing this type of activity are known as irreversible enzyme inhibitors.
Explanation of Solution
The Enzyme contains an active site where the substrate molecule comes and binds with the enzyme for the catalysis. The active site has complementary elements showing attraction forces in order to binds the substrate molecule to it. In the induced fit model, it was assumed that the bonding of substrate with an enzyme is reversible. That is it will leave the active site after the work is done. Reversible enzyme inhibitors are the molecules similar to substrate molecule having the same kind of atom or groups present on the outside that is complementary to the attraction forces to occur at the active site. In this way, inhibitor blocks the active site available for the substrate and inhibits the biochemical reaction. Sometimes it has been found that substrate remains bonded to an active site that is its bonding is irreversible. The compounds performing this kind of activity are known as irreversible enzyme inhibitors.
The terms reversible enzyme inhibitors and irreversible enzyme inhibitors are distinguished in the scientific terms rightfully above.
(d)
Interpretation:
The terms
Concept introduction:
Glucose is a sugar molecule also known as carbohydrates. It is a six carbon aldose sugar which means it contains
Answer to Problem 59E
The
Explanation of Solution
The two forms of glucose,
The structures of both forms of glucose are shown below.
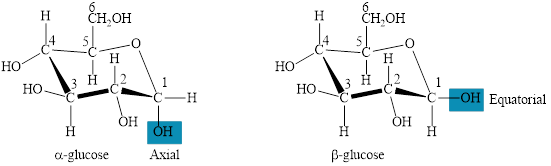
Figure 4
The terms
(e)
Interpretation:
The terms
Concept introduction:
Glucose is a sugar molecule also known as carbohydrates. It is a six carbon aldose sugar which means it contains aldehyde and hydroxide groups. Glucose has two forms
Answer to Problem 59E
The
Explanation of Solution
The polymers of glucose are starch and cellulose. The cellulose is a polymer of
The structure of cellulose with
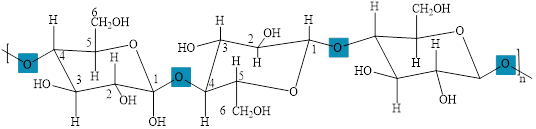
Figure 5
The structure of starch with
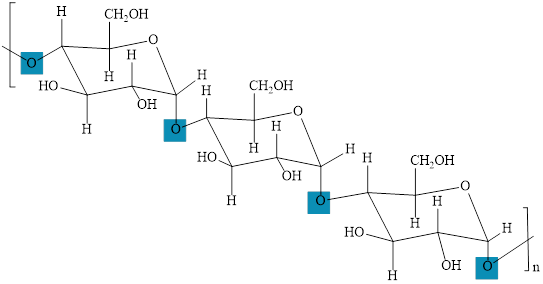
Figure 6
The terms
(f)
Interpretation:
The terms fats, oils are to be distinguished in the scientific terms.
Concept introduction:
Lipids are the water-insoluble compounds found in the living organisms. Lipids are parted into three classes (1) Fats, oils and phospholipids, (2) waxes, and (3) steroids.
All these compounds are insoluble in polar solvents like water because of the large presence of hydrophobic groups in them.
Answer to Problem 59E
The structure of fats and oils are the same. The difference between them is the state they acquire at room temperature. The fats are solid and oils are liquid at room temperature.
Explanation of Solution
Fats and oils are the triesters of long-chain carboxylic acids known as fatty acids. The ester is formed from simple alcohol glycerol. The fats and oils are also referred to as triacylglycerols. The fats at room temperature are solid while the oils are liquid at room temperature.
The terms fats, oils are distinguished in the scientific terms rightfully above.
(g)
Interpretation:
The terms saturated fatty acids, unsaturated fatty acids are to be distinguished in the scientific terms.
Concept introduction:
Fatty acids are the long-chain carboxylic acids. There may a saturated group attached to a carboxylic acid group or unsaturated group. On the basis of that fatty acids are of two types: saturated fatty acids and unsaturated fatty acids.
Answer to Problem 59E
Saturated fatty acids are compounds that do not have a double bond or have a fully saturated bond between carbon atoms. Unsaturated fatty acids contain one or more double bonds between the carbon atoms. Unsaturated fatty acids are liquid at room temperature, whereas saturated fatty acids are solid at room temperature. Unsaturated acids are more useful to the body than saturated fatty acids.
Explanation of Solution
Saturated fatty acids are the compounds having no double bond or have the fully saturated bonds between carbon atoms. Unsaturated fatty acids contain one or more double bond between the carbon atoms. Unsaturated fatty acids are liquid at room temperature while saturated fatty acids are solid at room temperature. Unsaturated are more beneficial for the body rather than saturated fatty acids.
The terms saturated fatty acids, unsaturated fatty acids are distinguished in the scientific terms rightfully above.
(h)
Interpretation:
The terms triacylglycerols, phospholipids are to be distinguished in the scientific terms.
Concept introduction:
Lipids are the water-insoluble compounds found in the living organisms. Lipids are parted into three classes (1) Fats, oils and phospholipids, (2) waxes, and (3) steroids.
All these compounds are insoluble in polar solvents like water because of the large presence of hydrophobic groups in them.
Answer to Problem 59E
Fats and oils are the triesters of long-chain carboxylic acids known as fatty acids. The ester is formed from simple glycerol alcohol. The fats and oils are also referred to as triacylglycerols. Phospholipids are also the same as triacylglycerols, but their backbone is an alcohol with two fatty acids and a phosphate group instead of glycerol as the backbone and three fatty acids in triacylglycerols.
Explanation of Solution
Fats and oils are the triesters of long-chain carboxylic acids known as fatty acids. The ester is formed with simple alcohol glycerol. The fats and oils are also known as triacylglycerols. Phospholipids are also same as triacylglycerols but their backbone is an alcohol having two fatty acids and phosphate group instead of glycerol as a backbone and three fatty acids in triacylglycerols.
The terms triacylglycerols, phospholipids are distinguished in the scientific terms rightfully above.
(i)
Interpretation:
The terms triacylglycerols, waxes are to be distinguished in the scientific terms
Concept introduction:
Lipids are the water-insoluble compounds found in the living organisms. Lipids are parted into three classes (1) Fats, oils and phospholipids, (2) waxes, and (3) steroids.
All these compounds are insoluble in polar solvents like water because of the large presence of hydrophobic groups in them.
Answer to Problem 59E
Fats and oils are the triesters of long-chain carboxylic acids known as fatty acids. The ester is formed with simple alcohol glycerol. Fats and oils are also known as triacylglycerols. Waxes are also ester, but with a long carbon chain monohydroxy alcohol.
Explanation of Solution
Fats and oils are the triesters of long-chain carboxylic acids known as fatty acids. The ester is formed with simple alcohol glycerol. The fats and oils are also known as triacylglycerols. Waxes are also ester but with a monohydroxyl alcohol with a long carbon chain.
The terms triacylglycerols, waxes are distinguished in the scientific terms rightfully above.
(j)
Interpretation:
The terms waxes, steroids are to be distinguished in the scientific terms
Concept introduction:
Lipids are the water-insoluble compounds found in the living organisms. Lipids are parted into three classes (1) Fats, oils and phospholipids, (2) waxes, and (3) steroids.
All these compounds are insoluble in polar solvents like water because of the large presence of hydrophobic groups in them.
Answer to Problem 59E
Waxes are the esters of fatty acids with a long carbon chain of monohydroxy alcohol. Steroids differ greatly from waxes. First, they do not contain an ester group. Second is that steroid contains a fused system of four rings. Three are six-carbon rings and one is five-carbon rings.
Explanation of Solution
Waxes are the esters of fatty acids with a long carbon chain monohydroxy alcohol. Steroids are very different from waxes. First, they do not contain any ester group. Second is steroid contains a four ring fused system. Three are six carbon and one is five carbon ring. The structure of one of the steroids cholesterol is shown below.
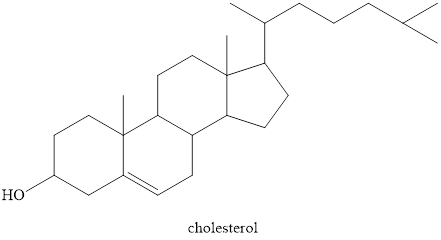
Figure 7
The terms waxes and steroids are distinguished in the scientific terms rightfully above.
(k)
Interpretation:
The terms nucleoside and
Concept introduction:
Nucleotides are the monomers of
Answer to Problem 59E
Nucleic acids are nucleotide polymers. Nucleotides and nucleosides differ from each other in such a way that nucleoside does not contain any phosphate group associated with sugar. A nucleoside is merely a combination of base-sugar. Nucleotides are a combination of phosphate and base-sugar.
Explanation of Solution
Nucleic acids are the polymer of nucleotides. Nucleotides and nucleoside differ from each other in a way that nucleoside does not contain any phosphate group linked to sugar. A nucleoside is just a base-sugar combination. Nucleotides are phosphate and base-sugar combination.
The terms nucleoside and nucleotide are distinguished in the scientific terms rightfully above.
(l)
Interpretation:
The terms thymine and uracil are to be distinguished in the scientific terms
Concept introduction:
DNA and RNA are the polymers of nucleotides. Nucleotides consist of three parts (a) cyclic nitrogenous base (b) a sugar (ribose or deoxyribose sugar) (c) phosphate group.
The cyclic nitrogenous bases are of two types (a) Purines (b) Pyrimidines.
Answer to Problem 59E
Thymine and uracil are both a pyrimidine nitrogen base consisting of a monocyclic ring. Their structure is very comparable to each other, the only distinction is that in the thymine there is one additional methyl group. Thymine is found only in DNA and Uracil is found only in DNA. These are the two cyclic nitrogen bases that differ in DNA and RNA.
Explanation of Solution
Thymine and uracil are both pyrimidine nitrogenous base consists of a monocyclic ring. Their structure is very similar to each other the only difference is there is one extra methyl group in the thymine. Thymine is only found in DNA and Uracil is only found in DNA. These are the two cyclic nitrogenous bases which are different in DNA and RNA.
The structures of thymine and uracil are given below.
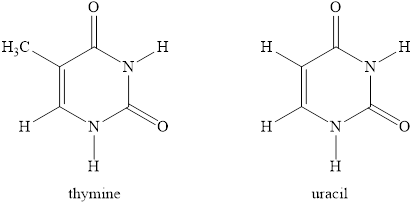
Figure 8
The terms thymine and uracil are distinguished in the scientific terms rightfully above.
(m)
Interpretation:
The terms
Concept introduction:
Secondary structure of proteins consists of a helical structure known as
Answer to Problem 59E
The
Explanation of Solution
The
The structure of
Figure 9
The structure of double helix is shown below.

Figure 10
The terms
Want to see more full solutions like this?
Chapter 22 Solutions
Introductory Chemistry: An Active Learning Approach
- Macmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forward
- If I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forward
- Predict the major organic product(s) for the following reactions.arrow_forwardProvide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forward
- If I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forwardIf I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,  Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





