
(a)
Interpretation: Using a different type of reactions, 1-hexanol is to be prepared from hexyl amine, heptyl amine and pentyl amine
Concept Introduction: A number of transformations are used to prepare 1-hexanol. Some of them are listed as follows:
- a) Alcohol on treatment with phosphorous tribromide gives alkyl bromide
- b) Alkyl bromide in azide synthesis produces primary amine
- c) Alkyl halide on treatment with sodium cyanide gives alkyl cyanide
- d) Cyanide on reduction gives alkyl amine with an increment of one carbon atom skeleton
- e)
Alkene on ozonolysis produces carbonyl compounds - f)
Alkyl halides with strong base gives alkene - g) Sodium cyanoborohydride is a strong reducing agent than sodium borohydride. It reduces the carbonyl group into amine group in a rapid way. So, it is called as reductive amination reactions.
Aldehyde orketone group is reacted with ammonia in the presence of sodium cyanoborohydride as a reducing agent and a proton source in the reaction medium to produce primaryamines .
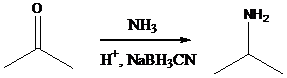
Using these concepts, we can transfer 1-hexanol into the given compounds.
(b)
Interpretation: Using a different type of reactions, 1-hexanol is to be prepared from hexyl amine, heptyl amine and pentyl amine
Concept Introduction: A number of transformations are used to prepare 1-hexanol. Some of them are listed as follows:
- a) Alcohol on treatment with phosphorous tribromide gives alkyl bromide
- b) Alkyl bromide in azide synthesis produces primary amine
- c) Alkyl halide on treatment with sodium cyanide gives alkyl cyanide
- d) Cyanide on reduction gives alkyl amine with an increment of one carbon atom skeleton
- e) Alkene on ozonolysis produces carbonyl compounds
- f) Alkyl halides with strong base gives alkene
- g) Sodium cyanoborohydride is a strong reducing agent than sodium borohydride. It reduces the carbonyl group into amine group in a rapid way. So, it is called as reductive amination reactions. Aldehyde or ketone group is reacted with ammonia in the presence of sodium cyanoborohydride as a reducing agent and a proton source in the reaction medium to produce primary amines.
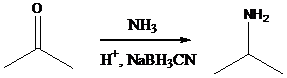
Using these concepts, we can transfer 1-hexanol into the given compounds.
(c)
Interpretation: Using a different type of reactions, 1-hexanol is to be prepared from hexyl amine, heptyl amine and pentyl amine
Concept Introduction: A number of transformations are used to prepare 1-hexanol. Some of them are listed as follows:
- a) Alcohol on treatment with phosphorous tribromide gives alkyl bromide
- b) Alkyl bromide in azide synthesis produces primary amine
- c) Alkyl halide on treatment with sodium cyanide gives alkyl cyanide
- d) Cyanide on reduction gives alkyl amine with an increment of one carbon atom skeleton
- e) Alkene on ozonolysis produces carbonyl compounds
- f) Alkyl halides with strong base gives alkene
- g) Sodium cyanoborohydride is a strong reducing agent than sodium borohydride. It reduces the carbonyl group into amine group in a rapid way. So, it is called as reductive amination reactions. Aldehyde or ketone group is reacted with ammonia in the presence of sodium cyanoborohydride as a reducing agent and a proton source in the reaction medium to produce primary amines.
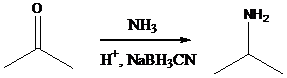
Using these concepts, we can transfer 1-hexanol into the given compounds.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- What does the phrase 'fit for purpose' mean in relation to analytical chemistry? Please provide examples too.arrow_forwardFor each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects Resonance Effects Overall Electron-Density × NO2 ○ donating O donating O withdrawing O withdrawing O electron-rich electron-deficient no inductive effects O no resonance effects O similar to benzene E [ CI O donating withdrawing O no inductive effects Explanation Check ○ donating withdrawing no resonance effects electron-rich electron-deficient O similar to benzene © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardUnderstanding how substituents activate Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation HN NH2 Check X (Choose one) (Choose one) (Choose one) (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Aarrow_forward
- Identifying electron-donating and electron-withdrawing effects on benzene For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Inductive Effects Resonance Effects Overall Electron-Density Molecule CF3 O donating O donating O withdrawing O withdrawing O no inductive effects O no resonance effects electron-rich electron-deficient O similar to benzene CH3 O donating O withdrawing O no inductive effects O donating O withdrawing Ono resonance effects O electron-rich O electron-deficient O similar to benzene Explanation Check Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward* Hint: Think back to Chem 1 solubility rules. Follow Up Questions for Part B 12. What impact do the following disturbances to a system at equilibrium have on k, the rate constant for the forward reaction? Explain. (4 pts) a) Changing the concentration of a reactant or product. (2 pts) b) Changing the temperature of an exothermic reaction. (2 pts) ofarrow_forwardDraw TWO general chemical equation to prepare Symmetrical and non-Symmetrical ethers Draw 1 chemical reaction of an etherarrow_forward
- Give the chemical equation for the preparation of: -Any aldehyde -Any keytonearrow_forward+ C8H16O2 (Fatty acid) + 11 02 → 8 CO2 a. Which of the above are the reactants? b. Which of the above are the products? H2o CO₂ c. Which reactant is the electron donor? Futty acid d. Which reactant is the electron acceptor? e. Which of the product is now reduced? f. Which of the products is now oxidized? 02 #20 102 8 H₂O g. Where was the carbon initially in this chemical reaction and where is it now that it is finished? 2 h. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward→ Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





