
Concept explainers
(a)
Interpretation:
The synthesis of
Concept introduction:
In
Answer to Problem 22.82AP
The synthesis of
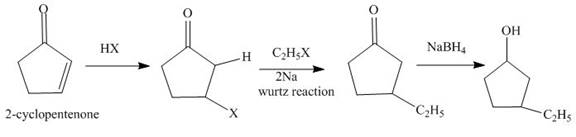
Explanation of Solution
The reaction of
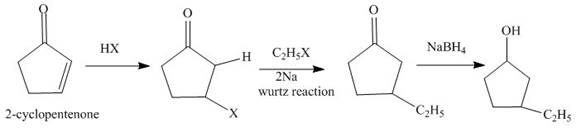
Figure 1
The synthesis of
(b)
Interpretation:
The synthesis of
Concept introduction:
In organic chemistry, the class of functional group which is derived from conjugated diene by replacing one double bond with a carbonyl group. The dienone compound is in conjugation to each other.
Answer to Problem 22.82AP
The synthesis of
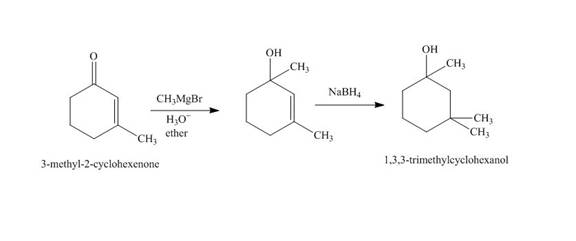
Explanation of Solution
The reaction of
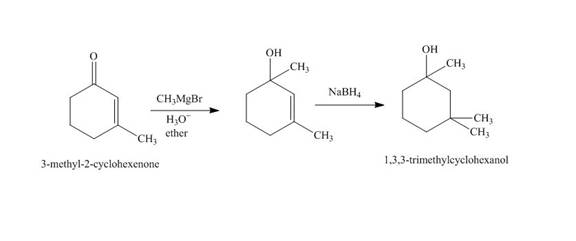
Figure 2
The synthesis of
(c)
Interpretation:
The synthesis of
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 22.82AP
The synthesis of
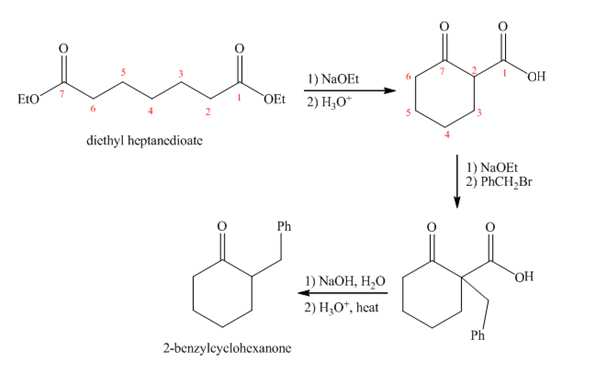
Explanation of Solution
Diethyl heptanedioate in presence of

Figure 3
The synthesis of
(d)
Interpretation:
The synthesis of
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 22.82AP
The synthesis of
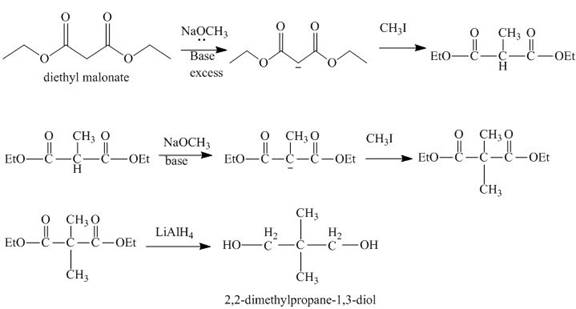
Explanation of Solution
The reaction of diethyl malonate in presence of base and then

Figure 4
The synthesis of
(e)
Interpretation:
The synthesis of
Concept introduction:
An ester is a derivative of
Answer to Problem 22.82AP
The synthesis of
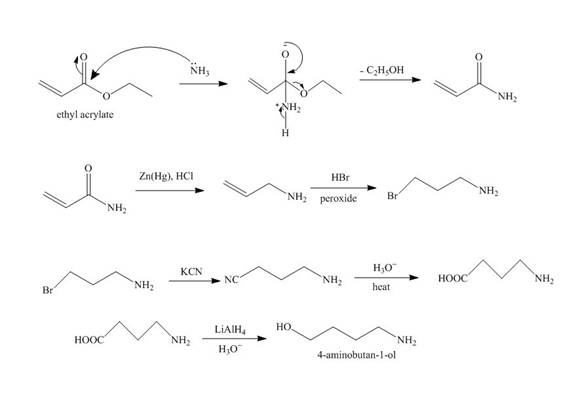
Explanation of Solution
The reaction of ethyl acrylate with ammonia forms amide derivative. It undergoes reduction reaction with

Figure 5
The synthesis of
(f)
Interpretation:
The synthesis of
Concept introduction:
In organic chemistry, the compound with the molecular formula
Answer to Problem 22.82AP
The synthesis of
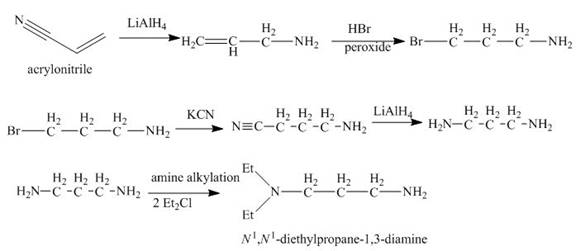
Explanation of Solution
The reaction of acrylonitrile with
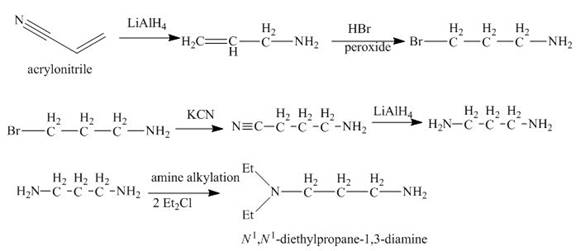
Figure 6
The synthesis of
(g)
Interpretation:
The synthesis of
Concept introduction:
Carboxylic acid is a class of organic compound contains a
Answer to Problem 22.82AP
The synthesis of
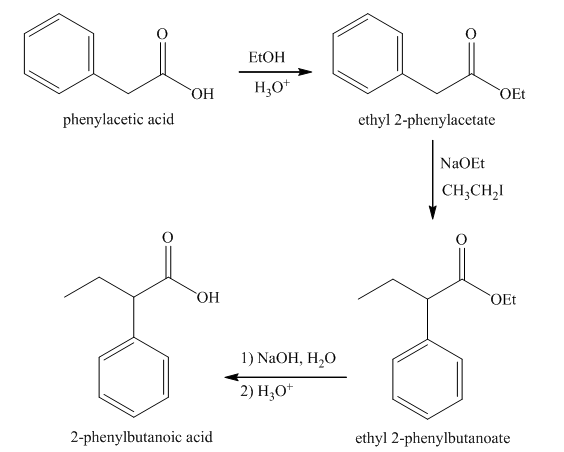
Explanation of Solution
Esterification of phenylacetic acid forms ethyl

Figure 7
The synthesis of
(h)
Interpretation:
The synthesis of
Concept introduction:
In Aldol condensation reaction, the base abstracts an acidic proton from the
Answer to Problem 22.82AP
The synthesis of
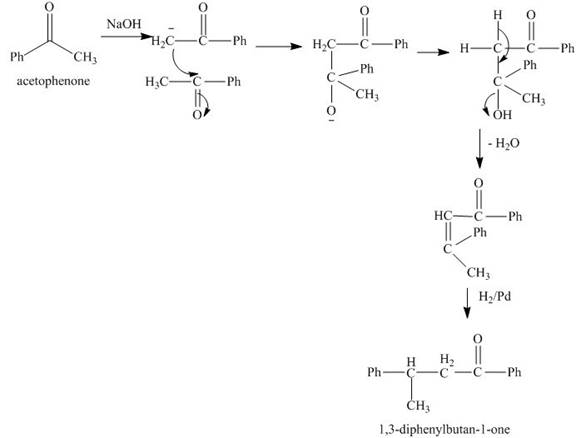
Explanation of Solution
The reaction of acetophenone in presence of base abstracts a

Figure 8
The synthesis of
(i)
Interpretation:
The synthesis of deuterium substituted diphenylethanol from phenylacetic acid is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 22.82AP
The synthesis of di-deuterium substituted diphenyl ethanol from phenylacetic acid is shown below.
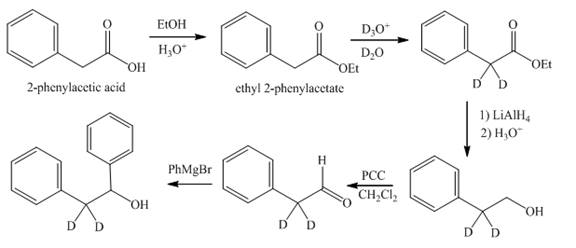
Explanation of Solution
Reaction of

Figure 9
The synthesis of deuterium substituted diphenyl ethanol from phenylacetic acid is shown in Figure 9.
(j)
Interpretation:
The synthesis of tetra-deuterium substituted product from cyclohexanone is to be stated.
Concept introduction:
In organic chemistry, the carbonyl compound is a class of functional group which contains a
Answer to Problem 22.82AP
The synthesis of tetra-deuterium substituted product from cyclohexanone is shown below.
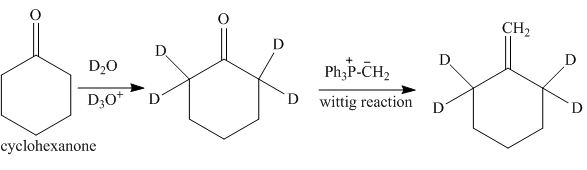
Explanation of Solution
The reaction of cyclohexanone with

Figure 10
The synthesis of tetra-deuterium substituted from cyclohexanone is shown in Figure 10.
(k)
Interpretation:
The synthesis of
Concept introduction:
Friedel Craft acylation is an electrophilic aromatic substitution reaction. In this reaction, the synthesis of the monoacylated product takes place from the reaction between aromatic rings and acyl chlorides. The organic reaction in which an organohalide is reacted with alcohols or phenols to form ethers is Willamson synthesis reaction.
Answer to Problem 22.82AP
The synthesis of
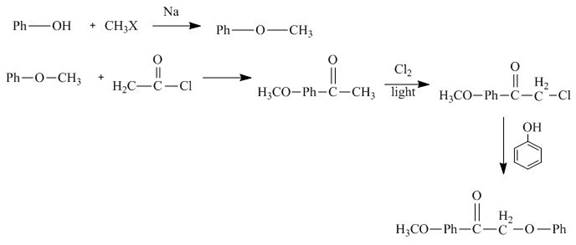
Explanation of Solution
The reaction of phenol with methyl halide forms methylphenyl ether. It reacts with acetyl chloride followed by chlorination in sunlight. Then it reacts with phenol to give the desired product. The synthesis of

Figure 11
The synthesis of
(l)
Interpretation:
The synthesis of
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 22.82AP
The synthesis of
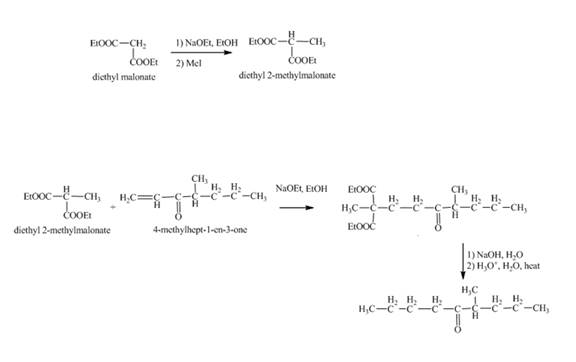
Explanation of Solution
Methylation of diethyl malonate in the presence of sodium ethoxide and

Figure 12
The synthesis of
(m)
Interpretation:
The synthesis of
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 22.82AP
The synthesis of
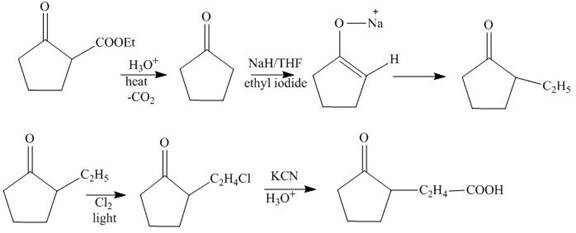
Explanation of Solution
The hydrolysis reaction of

Figure13
The synthesis of
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- Hi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forwardHi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forward
- Indicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forwardDraw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forward
- Draw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forwardExplain how substitutions at the 5-position of barbituric acid increase the compound's lipophilicity.arrow_forward
- Explain how substitutions at the 5-position of phenobarbital increase the compound's lipophilicity.arrow_forwardName an interesting derivative of barbituric acid, describing its structure.arrow_forwardBriefly describe the synthesis mechanism of barbituric acid from the condensation of urea with a β-diketone.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

