
A 1.00-mol sample of an ideal gas (γ = 1.40) is carried through the Carnot cycle described in Figure 22.11. At point A, the pressure is 25.0 atm and the temperature is 600 K. At point C, the pressure is 1.00 atm and the temperature is 400 K. (a) Determine the pressures and volumes at points A, B, C, and D. (b) Calculate the net work done per cycle.
(a)
The pressure and volume at points
Answer to Problem 22.81CP
For the point
Explanation of Solution
Given data: The number of mole is
Consider the figure of Carnot cycle,
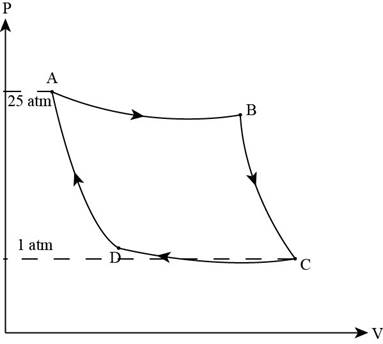
Figure (1)
From the figure, it is clear that the process from
Apply the ideal gas equation for volume at
Here,
The value of ideal gas constant is
Substitute
Thus, the volume at point
Apply the ideal gas equation for volume at
Here,
Substitute
Thus, the volume at point
For the adiabatic process:
The condition is,
Here,
Substitute
Thus, the volume of gas at point
The formula for the pressure at point
Substitute
Thus, the pressure at point
For the adiabatic process:
The condition is,
Here,
Substitute
Thus, the volume of gas at point
The formula for the pressure at point
Substitute
Conclusion:
Therefore, For the point
(b)
The net work done per cycle.
Answer to Problem 22.81CP
The net work done per cycle is
Explanation of Solution
The formula for the net work done per cycle is,
Substitute
Conclusion:
Therefore, the net work done per cycle is
Want to see more full solutions like this?
Chapter 22 Solutions
EBK PHYSICS FOR SCIENTISTS AND ENGINEER
- The compression ratio of an Otto cycle as shown in Figure 21.12 is VA/VB = 8.00. At the beginning A of the compression process, 500 cm3 of gas is at 100 kPa and 20.0C. At the beginning of the adiabatic expansion, the temperature is TC = 750C. Model the working fluid as an ideal gas with = 1.40. (a) Fill in this table to follow the states of the gas: (b) Fill in this table to follow the processes: (c) Identify the energy input |Qh|, (d) the energy exhaust |Qc|, and (e) the net output work Weng. (f) Calculate the efficiency. (g) Find the number of crankshaft revolutions per minute required for a one-cylinder engine to have an output power of 1.00 kW = 1.34 hp. Note: The thermodynamic cycle involves four piston strokes.arrow_forwardOne mole of an ideal gas does 3 000 J of work on its surroundings as it expands isothermally to a final pressure of 1.00 atm and volume of 25.0 L. Determine (a) the initial volume and (b) the temperature of the gas.arrow_forwardAn ideal gas with specific heat ratio confined to a cylinder is put through a closed cycle. Initially, the gas is at Pi, Vi, and Ti. First, its pressure is tripled under constant volume. It then expands adiabatically to its original pressure and finally is compressed isobarically to its original volume. (a) Draw a PV diagram of this cycle. (b) Determine the volume at the end of the adiabatic expansion. Find (c) the temperature of the gas at the start of the adiabatic expansion and (d) the temperature at the end of the cycle. (e) What was the net work done on the gas for this cycle?arrow_forward
- A sample of a monatomic ideal gas is contained in a cylinder with a piston. Its state is represented by the dot in the PV diagram shown in Figure OQ18.9. Arrows A through E represent isobaric, isothermal, adiabatic, and isovolumetric processes that the sample can undergo. In each process except D, the volume changes by a factor of 2. All five processes are reversible. Rank the processes according to the change in entropy of the gas from the largest positive value to the largest-magnitude negative value. In your rankings, display any cases of equality. Figure OQ18.9arrow_forwardThe arrow OA in the PV diagram shown in Figure OQ22.11 represents a reversible adiabatic expansion of an ideal gas. The same sample of gas, starting from the same state O. now undergoes an adiabatic free expansion to the same final volume. What point on the diagram could represent the final state of the gas? (a) the same point A as for the reversible expansion (b) point B (c) point C (d) any of those choices (e) none of those choicesarrow_forwardAir (a diatomic ideal gas) at 27.0C and atmospheric pressure is drawn into a bicycle pump (Figure P17.53) that has a cylinder with an inner diameter of 2.50 cm and length 50.0 cm. The downstroke adiabatically compresses the air, which reaches a gauge pressure of 8.00 105 Pa before entering the tire. We wish to investigate the temperature increase of the pump. (a) What is the initial volume of the air in the pump? (b) What is the number of moles of air in the pump? (c) What is the absolute pressure of the compressed air? (d) What is the volume of the compressed air? (e) What is the temperature of the compressed air? (f) What is the increase in internal energy of the gas during the compression? What If? The pump is made of steel that is 2.00 mm thick. Assume 4.00 cm of the cylinders length is allowed to come to thermal equilibrium with the air. (g) What is the volume of steel in this 4.00-cm length? (h) What is the mass of steel in this 4.00-cm length? (i) Assume the pump is compressed once. After the adiabatic expansion, conduction results in the energy increase in part (f) being shared between the gas and the 4.00-cm length of steel. What will be the increase in temperature of the steel after one compression? Figure P17.53arrow_forward
- Of the following, which is not a statement of the second law of thermodynamics? (a) No heat engine operating in a cycle can absorb energy from a reservoir and use it entirely to do work, (b) No real engine operating between two energy reservoirs can be more efficient than a Carnot engine operating between the same two reservoirs, (c) When a system undergoes a change in state, the change in the internal energy of the system is the sum of the energy transferred to the system by heat and the work done on the system, (d) The entropy of the Universe increases in all natural processes, (e) Energy will not spontaneously transfer by heat from a cold object to a hot object.arrow_forwardFigure P22.73 illustrates the cycle ABCA for a 2.00-mol sample of an ideal diatomic gas, where the process CA is a reversible isothermal expansion. What is a. the net work done by the gas during one cycle? b. How much energy is added to the gas by heat during one cycle? c. How much energy is exhausted from the gas by heat during one cycle? d. What is the efficiency of the cycle? e. What would be the efficiency of a Carnot engine operated between the temperatures at points A and B during each cycle?arrow_forward1 mole of monatomic ideal gas has been taken through a cycle as shown in the PV diagram. Vc = 8Vb. Vb = 0.001 m3 and Pb = 106 Pa. The process bc is an adiabatic process. Find the net work done by the gas during each cycle.arrow_forward
- A certain gasoline engine is modeled as a monatomic ideal gas undergoing an Otto cycle, represented by the p-V diagram shown in the figure. The initial pressure, volume, and temperature are p1 = 1.05 × 105 Pa, V1 = 0.035 m3, and T1 = 290 K, respectively. a)The first step in the Otto cycle is adiabatic compression. Enter an expression for the work performed on the gas during the first step, in terms of V1, V2, and p1. b) Calculate the work performed on the gas during the first step, in joules, for V2 = V1/9.4. c)Calculate the temperature of the gas, in kelvins, at the end of the first step.arrow_forwardA certain gasoline engine is modeled as a monatomic ideal gas undergoing an Otto cycle, represented by the p-V diagram shown in the figure. The initial pressure, volume, and temperature are p1 = 0.95 × 105 Pa, V1 = 0.045 m3, and T1 = 295 K, respectively.A) The first step in the Otto cycle is adiabatic compression. Enter an expression for the work performed on the gas during the first step, in terms of V1, V2, and p1 B) Calculate the work performed on the gas during the first step, in joules, for V2=V1/9.7 C) The second step in the otto cycle is isochoric heating, calculate the heat absorbed by the gas during this process, in joules, if the temperature is increased so that T3=1.4*T2 (T2=1341.74K) D) Calculate the pressure at the end of the isochoric heating step, in pascals, to three significant figures E) The thid step in the otto cycle is adibiatic expansion, which brings the volume back to its initial value, calculate the work performed on the gas, in joules, during the third step.…arrow_forwardThe pV diagram in (Figure 1) shows the cycle followed by the gas in an ideal-gas heat engine. 260 J of heat energy flow into gas from the hot reservoir during process 1→2. How much work is done during one cycle?arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





