
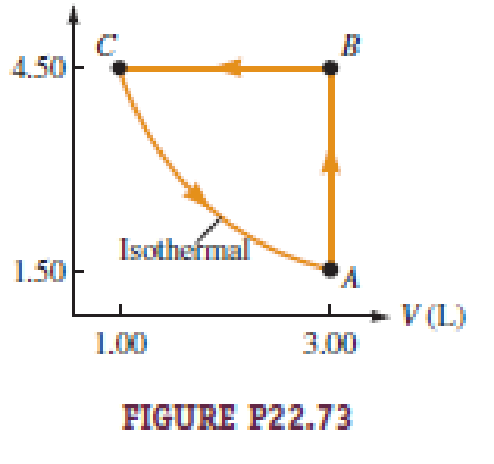
Figure P22.73 illustrates the cycle ABCA for a 2.00-mol sample of an ideal diatomic gas, where the process CA is a reversible isothermal expansion. What is a. the net work done by the gas during one cycle? b. How much energy is added to the gas by heat during one cycle? c. How much energy is exhausted from the gas by heat during one cycle? d. What is the efficiency of the cycle? e. What would be the efficiency of a Carnot engine operated between the temperatures at points A and B during each cycle?
(a)
The net work done by the gas during one cycle.
Answer to Problem 73PQ
The net work done by the gas during one cycle is
Explanation of Solution
The work done by the gas along AB is
Write the expression to calculate the work done by the gas along BC.
Here,
Write the expression to calculate the work done by the gas along CA.
Here,
Write the expression to calculate the total work done by the gas.
Here, W is the total work done by the gas.
Conclusion:
Substitute
Substitute
Substitute
Therefore, the net work done by the gas during one cycle is
(b)
The heat added in one cycle.
Answer to Problem 73PQ
The heat added in one cycle is
Explanation of Solution
For the diatomic gases, the specific heat capacity at constant volume is
The temperature at A and C of the gas is same.
Write the expression to calculate the temperature of the gas at A and C.
Here, T is the temperature of the gas at A and C, P is the pressure at A, V is the pressure at A n is the number of moles and R is the universal gas constant.
Write the expression to calculate the temperature at B.
Here,
Write the expression to calculate the energy added to the system.
Here, Q is the energy added to the system,
Substitute
Conclusion:
Substitute
Substitute
Substitute
Therefore, the heat added in one cycle is
(c)
The energy exhausted from the gas.
Answer to Problem 73PQ
The energy exhausted from the gas is
Explanation of Solution
For the diatomic gases, the specific heat capacity at constant pressure is
Write the expression to calculate the heat exhausted from the gas.
Here,
Substitute
Conclusion:
Substitute
Therefore, the energy exhausted from the gas is
(d)
The efficiency of the cycle.
Answer to Problem 73PQ
The efficiency of the cycle is
Explanation of Solution
Write the expression to calculate the efficiency of one cycle.
Here, e is the efficiency.
Conclusion:
Substitute
Therefore, the efficiency of the cycle is
(e)
The efficiency of the Carnot engine.
Answer to Problem 73PQ
The efficiency of the Carnot engine is
Explanation of Solution
Write the expression to calculate the efficiency of the Carnot engine.
Here,
Conclusion:
Substitute
Therefore, the efficiency of the Carnot engine is
Want to see more full solutions like this?
Chapter 22 Solutions
Physics for Scientists and Engineers: Foundations and Connections
- A thrown brick hits a window, but doesn't break it. Instead it reverses direction and ends down on the ground below the window. Since the brick didn't break the glass, we know: О The force of the brick on the glass > the force of the glass on the brick. О The force of the brick on the glass the force of the glass on the brick. = О The force of the brick on the glass < the force of the glass on the brick. О The brick didn't slow down as it broke the glass.arrow_forwardAlexandra (wearing rubber boots for traction) is attempting to drag her 32.6-kg Golden Retriever across the smooth ice by applying a horizontal force. What force must she apply to move the dog with a constant speed of 0.950 m/s? ☐ 31.0 lb. ☐ 319 kg. ○ Zero. 32.6 kg.arrow_forwardThe figure shows a graph of the acceleration of an object as a function of the net force acting on it. The mass of this object, in grams, is closest to 11 a(m/s²) 8.0+ 6.0- 4.0- 2.0- 0+ F(N) 0.00 0.50 1.00 ☐ 130 ○ 8000 ☐ 89arrow_forward
- Values that are within standard deviations represent measurements that are considered to be near the true value. Review the data from the lab and determine whether your data is within standard deviations. Report, using numerical values, whether your data for each angle is within standard deviations. An acceptable margin of error typically falls between 4% and 8% at the 95% confidence level. Review your data for each angle to determine whether the margin of error is within an acceptable range. Report with numerical values, whether your data for each angle is within an acceptable margin of error. Can you help explain what my data means in terms of the standard deviation and the ME? Thanks!arrow_forwardA sinusoidal wave is propagating along a stretched string that lies along the x-axis. The displacement of the string as a function of time is graphed in (Figure 1) for particles at x = 0 and at x = 0.0900 m. You are told that the two points x = 0 and x = 0.0900 m are within one wavelength of each other. If the wave is moving in the +x-direction, determine the wavelength. If instead the wave is moving in the -x-direction, determine the wavelength. Please show all stepsarrow_forwardYou are designing a two-string instrument with metal strings 35.0 cm long, as shown in (Figure 1). Both strings are under the same tension. String S1 has a mass of 8.30 g and produces the note middle C (frequency 262 Hz ) in its fundamental mode. What should be the tension in the string? What should be the mass of string S2 so that it will produce A-sharp (frequency 466 Hz ) as its fundamental? To extend the range of your instrument, you include a fret located just under the strings but not normally touching them. How far from the upper end should you put this fret so that when you press S1 tightly against it, this string will produce C-sharp (frequency 277 Hz ) in its fundamental? That is, what is x in the figure? If you press S2 against the fret, what frequency of sound will it produce in its fundamental?arrow_forward
- Please solve and answer the problem correctly please. Thank you!!arrow_forwardPlease help explain this. The experiment without the sandpaper had a 5% experimental error, with sandpaper it is 9.4%. Would the explaination be similar to the experiment without sandpaper? Thanks!arrow_forwardA sinusoidal wave with wavelength 0.400 m travels along a string. The maximum transverse speed of a point on the string is 3.00 m/s and the maximum transverse acceleration is 8.10×104m/s2. What is the propagation speed v of the wave? What is the amplitude A of the wave?arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





