
Chemistry, Books a la Carte Edition and Modified Mastering Chemistry with Pearson eText & ValuePack Access Card (7th Edition)
7th Edition
ISBN: 9780134172514
Author: John E. McMurry
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 22, Problem 22.40CP
Interpretation Introduction
To determine:
To find out the oxidation state of oxygen in each oxide.
Interpretation Introduction
To Determine:
To find out these oxides is molecular, and which has an infinitely extended three dimensional structure.
Interpretation Introduction
To Determine:
To find out these oxides is likely to be a gas or liquid and which is likely to be high melting solid..
Interpretation Introduction
To Determine:
To find out the other elements in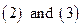
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2. Use Hess's law to calculate the AH
(in kJ) for:
rxn
CIF(g) + F2(g) →
CIF 3 (1)
using the following information:
2CIF(g) + O2(g) →
Cl₂O(g) + OF 2(g)
AH = 167.5 kJ
ΔΗ
2F2 (g) + O2(g) → 2 OF 2(g)
2C1F3 (1) + 202(g) → Cl₂O(g) + 3 OF 2(g)
о
=
= -43.5 kJ
AH = 394.1kJ
ci
Draw the major product(s) of the following reactions: (3 pts)
CH3
HNO3/H2SO4
HNO3/ H2SO4
OCH3
(1 pts)
Provide the product for the reaction
Chapter 22 Solutions
Chemistry, Books a la Carte Edition and Modified Mastering Chemistry with Pearson eText & ValuePack Access Card (7th Edition)
Ch. 22 - Prob. 22.1PCh. 22 - Prob. 22.2ACh. 22 - Prob. 22.3PCh. 22 - Prob. 22.4CACh. 22 - Prob. 22.5PCh. 22 - Prob. 22.6PCh. 22 - Prob. 22.7ACh. 22 - Prob. 22.8CPCh. 22 - Prob. 22.9ACh. 22 - Prob. 22.10P
Ch. 22 - Prob. 22.11PCh. 22 - Prob. 22.12PCh. 22 - Prob. 22.13PCh. 22 - Prob. 22.14PCh. 22 - Prob. 22.15PCh. 22 - Prob. 22.16PCh. 22 - Prob. 22.17CPCh. 22 - Prob. 22.18CACh. 22 - Prob. 22.19PCh. 22 - Prob. 22.20PCh. 22 - Prob. 22.21PCh. 22 - Conceptual PRACTICE 22.22 Look at the location of...Ch. 22 - Prob. 22.23ACh. 22 - Prob. 22.24CPCh. 22 - Prob. 22.25PCh. 22 - Prob. 22.26PCh. 22 - Prob. 22.27PCh. 22 - Prob. 22.28PCh. 22 - Prob. 22.29PCh. 22 - Prob. 22.30PCh. 22 - Prob. 22.31PCh. 22 - Prob. 22.32PCh. 22 - Prob. 22.33CPCh. 22 - Prob. 22.34CPCh. 22 - Prob. 22.35CPCh. 22 - Prob. 22.36CPCh. 22 - Prob. 22.37CPCh. 22 - Prob. 22.38CPCh. 22 - Prob. 22.39CPCh. 22 - Prob. 22.40CPCh. 22 - Prob. 22.41CPCh. 22 - Prob. 22.42CPCh. 22 - Prob. 22.43CPCh. 22 - Prob. 22.44CPCh. 22 - Prob. 22.45CPCh. 22 - Prob. 22.46CPCh. 22 - Prob. 22.47CPCh. 22 - Prob. 22.48SPCh. 22 - Prob. 22.49SPCh. 22 - Prob. 22.50SPCh. 22 - Prob. 22.51SPCh. 22 - Prob. 22.52SPCh. 22 - Prob. 22.53SPCh. 22 - Prob. 22.54SPCh. 22 - Prob. 22.55SPCh. 22 - Prob. 22.56SPCh. 22 - Prob. 22.57SPCh. 22 - Prob. 22.58SPCh. 22 - Prob. 22.59SPCh. 22 - Prob. 22.60SPCh. 22 - Prob. 22.61SPCh. 22 - Prob. 22.62SPCh. 22 - Prob. 22.63SPCh. 22 - Prob. 22.64SPCh. 22 - Prob. 22.65SPCh. 22 - Prob. 22.66SPCh. 22 - Prob. 22.67SPCh. 22 - Prob. 22.68SPCh. 22 - Prob. 22.69SPCh. 22 - Prob. 22.70SPCh. 22 - Prob. 22.71SPCh. 22 - Prob. 22.72SPCh. 22 - Prob. 22.73SPCh. 22 - Prob. 22.74SPCh. 22 - Prob. 22.75SPCh. 22 - Prob. 22.76SPCh. 22 - Prob. 22.77SPCh. 22 - Prob. 22.78SPCh. 22 - Prob. 22.79SPCh. 22 - Prob. 22.80SPCh. 22 - Prob. 22.81SPCh. 22 - Prob. 22.82SPCh. 22 - Prob. 22.83SPCh. 22 - Prob. 22.84SPCh. 22 - Prob. 22.85SPCh. 22 - Magnesium metal is produced by electrolysis of...Ch. 22 - Prob. 22.87SPCh. 22 - Prob. 22.88SPCh. 22 - Prob. 22.89SPCh. 22 - Prob. 22.90SPCh. 22 - Prob. 22.91SPCh. 22 - Prob. 22.92SPCh. 22 - Prob. 22.93SPCh. 22 - Prob. 22.94SPCh. 22 - Prob. 22.95SPCh. 22 - Prob. 22.96SPCh. 22 - Prob. 22.97SPCh. 22 - Prob. 22.98SPCh. 22 - Prob. 22.99SPCh. 22 - Prob. 22.100SPCh. 22 - Prob. 22.101SPCh. 22 - Prob. 22.102SPCh. 22 - Prob. 22.103SPCh. 22 - Prob. 22.104SPCh. 22 - Prob. 22.105SPCh. 22 - Prob. 22.106SPCh. 22 - Prob. 22.107SPCh. 22 - Prob. 22.108SPCh. 22 - Prob. 22.109SPCh. 22 - Prob. 22.110SPCh. 22 - Prob. 22.111SPCh. 22 - Prob. 22.112SPCh. 22 - Prob. 22.113SPCh. 22 - Prob. 22.114SPCh. 22 - Prob. 22.115SPCh. 22 - Prob. 22.116SPCh. 22 - Prob. 22.117SPCh. 22 - Prob. 22.118SPCh. 22 - Prob. 22.119SPCh. 22 - Prob. 22.120SPCh. 22 - Prob. 22.121SPCh. 22 - Prob. 22.122SPCh. 22 - Prob. 22.123SPCh. 22 - Prob. 22.124SPCh. 22 - Prob. 22.125SPCh. 22 - Prob. 22.126SPCh. 22 - Prob. 22.127SPCh. 22 - Prob. 22.128SPCh. 22 - Prob. 22.129SPCh. 22 - Prob. 22.130SPCh. 22 - Prob. 22.131SPCh. 22 - Prob. 22.132SPCh. 22 - Prob. 22.133SPCh. 22 - Prob. 22.134SPCh. 22 - Prob. 22.135SPCh. 22 - Prob. 22.136SPCh. 22 - Prob. 22.137SPCh. 22 - Prob. 22.138SPCh. 22 - Prob. 22.139SPCh. 22 - Prob. 22.140SPCh. 22 - Prob. 22.141SPCh. 22 - Prob. 22.142SPCh. 22 - Prob. 22.143SPCh. 22 - Prob. 22.144SPCh. 22 - Prob. 22.145SPCh. 22 - Prob. 22.146SPCh. 22 - Prob. 22.147SPCh. 22 - Prob. 22.148SPCh. 22 - Prob. 22.149SPCh. 22 - Prob. 22.150SPCh. 22 - Prob. 22.151SPCh. 22 - Prob. 22.152SPCh. 22 - Prob. 22.153SPCh. 22 - Prob. 22.154SPCh. 22 - Prob. 22.155SPCh. 22 - Prob. 22.156SPCh. 22 - Prob. 22.157SPCh. 22 - Prob. 22.158CPCh. 22 - Prob. 22.159CPCh. 22 - Prob. 22.160CPCh. 22 - Prob. 22.161CPCh. 22 - Prob. 22.162CPCh. 22 - Prob. 22.163CPCh. 22 - Prob. 22.164CPCh. 22 - Prob. 22.165CPCh. 22 - Prob. 22.166CPCh. 22 - Prob. 22.167CPCh. 22 - Prob. 22.168CPCh. 22 - Prob. 22.169CPCh. 22 - Prob. 22.170CPCh. 22 - Prob. 22.171CPCh. 22 - Prob. 22.172CPCh. 22 - Prob. 22.173CPCh. 22 - Prob. 22.174CPCh. 22 - Prob. 22.175MPCh. 22 - Prob. 22.176MPCh. 22 - Prob. 22.177MPCh. 22 - Prob. 22.178MPCh. 22 - Prob. 22.179MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the net ionic equation for the reaction between tin(IV) sulfide and nitric acid?arrow_forwardThe combustion of 28.8 g of NH3 consumes exactly _____ g of O2. 4 NH3 + 7 O2 ----> 4 NO2 + 6 H2Oarrow_forwardWhat is the molecular formula of the bond-line structure shown below OH HO ○ C14H12O2 ○ C16H14O2 ○ C16H12O2 O C14H14O2arrow_forward
- Check all molecules that are acids on the list below. H2CO3 HC2H3O2 C6H5NH2 HNO3 NH3arrow_forwardFrom the given compound, choose the proton that best fits each given description. a CH2 CH 2 Cl b с CH2 F Most shielded: (Choose one) Least shielded: (Choose one) Highest chemical shift: (Choose one) Lowest chemical shift: (Choose one) ×arrow_forwardConsider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 1H NMR spectrum? Note: A multiplet is considered one signal.arrow_forward
- For each of the given mass spectrum data, identify whether the compound contains chlorine, bromine, or neither. Compound m/z of M* peak m/z of M + 2 peak ratio of M+ : M + 2 peak Which element is present? A 122 no M + 2 peak not applicable (Choose one) B 78 80 3:1 (Choose one) C 227 229 1:1 (Choose one)arrow_forwardShow transformation from reactant to product, step by step. *see imagearrow_forwardCheck the box if the molecule contains the listed item. *See imagearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY