
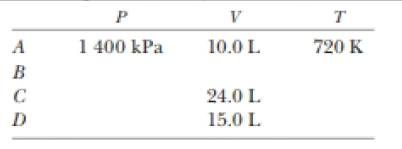
At point A in a Carnot cycle, 2.34 mol of a monatomic ideal gas has a pressure of 1 4000 kPa, a volume of 10.0 L, and a temperature of 720 K. The gas expands isothermally to point B and then expands adiabatically to point C, where its volume is 24.0 L. An isothermal compression brings it to point D, where its volume is 15.0 L. An adiabatic process returns the gas to point A. (a) Determine all the unknown pressures, volumes, and temperatures as you f ill in the following table:
(b) Find the energy added by heat, the work done by the engine, and the change in internal energy for each of the steps A → B, B → C, C → D, and D → A (c) Calculate the efficiency Wnet/|Qk|. (d) Show that the efficiency is equal to 1 - TC/TA, the Carnot efficiency.
(a)
The unknown pressures, volumes and the temperature in the table.
Answer to Problem 22.32P
The values of unknown pressures, volumes and the temperature in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Write the equation of adiabatic process
Here,
The value of
Substitute
Thus, the pressure of the gas at point
Write the ideal gas equation.
Here,
The value of gas constant is
Substitute
Thus, the temperature of the gas at point
In isothermal process, the temperature is constant.
For isothermal process
The temperature of the gas at point
Thus, the temperature of the gas at point
Write the ideal gas equation.
Here,
Substitute
Thus, the pressure of the gas at point
Write the equation of adiabatic process
Here,
Substitute
Thus, the volume of the gas at point
In isothermal process, the temperature is constant.
For isothermal process
The temperature of the gas at point
Thus, the temperature of the gas at point
Write the ideal gas equation.
Here,
Substitute
Thus, the pressure of the gas at point
Form a table and show the unknown value of pressures, volumes and temperatures.
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conclusion:
Therefore, the values of unknown pressures, volumes and the temperature in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(b)
The energy added by heat, work done by the engine and the change in internal energy for each of the steps
Answer to Problem 22.32P
The values of energy added by heat, work done by the engine and the change in internal energy for each of the steps in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
The process
Write the equation of change in temperature for process
Here,
Substitute
Thus, the change in internal energy for process
Write the equation of work done by the engine for process
Substitute
Thus, the work done by the engine for process
Write the equation of isothermal process
Substitute
Thus, the energy added by heat for process
Write the equation of change in temperature for process
Here,
The value of
Substitute
Substitute
Thus, the change in internal energy for process
The process
Thus, the energy added by heat for process
Write the equation of change in internal energy for process
Substitute
Thus, the work done by the engine for process
The process
Write the equation of change in temperature for process
Substitute
Thus, the change in internal energy for process
Write the equation of work done by the engine for process
Substitute
Thus, the work done by the engine for process
Write the equation of isothermal process
Substitute
Thus, the energy added by heat for process
Write the equation of change in temperature for process
Substitute
Substitute
Thus, the change in internal energy for process
The process
Thus, the energy added by heat for process
Write the equation of change in internal energy for process
Substitute
Thus, the work done by the engine for process
Form a table and show the value of energy added by heat, work done by the engine and the change in internal energy.
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conclusion:
Therefore, the values of energy added by heat, work done by the engine and the change in internal energy for each of the steps in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(c)
The value of efficiency
Answer to Problem 22.32P
The value of efficiency
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Calculate the net work done from the table is,
Write the equation for efficiency.
Here,
Substitute
The value of efficiency
Conclusion:
Therefore, the value of efficiency
(d)
To show: The efficiency is equal to the Carnot efficiency
Answer to Problem 22.32P
The efficiency is equal to the Carnot efficiency
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Write the equation for Carnot efficiency.
Here,
The value of
Substitute
Thus, the Carnot efficiency is
Write the equation for efficiency.
Substitute
The value of efficiency is
Conclusion:
Therefore, the efficiency is equal to the Carnot efficiency
Want to see more full solutions like this?
Chapter 22 Solutions
Physics for Scientists and Engineers, Technology Update (No access codes included)
- PROBLEM 2 A cube of mass m is placed in a rotating funnel. (The funnel is rotating around the vertical axis shown in the diagram.) There is no friction between the cube and the funnel but the funnel is rotating at just the right speed needed to keep the cube rotating with the funnel. The cube travels in a circular path of radius r, and the angle between the vertical and the wall of the funnel is 0. Express your answers to parts (b) and (c) in terms of m, r, g, and/or 0. (a) Sketch a free-body diagram for the cube. Show all the forces acting on it, and show the appropriate coordinate system to use for this problem. (b) What is the normal force acting on the cube? FN=mg58 (c) What is the speed v of the cube? (d) If the speed of the cube is different from what you determined in part (c), a force of friction is necessary to keep the cube from slipping in the funnel. If the funnel is rotating slower than it was above, draw a new free-body diagram for the cube to show which way friction…arrow_forwardCircular turns of radius r in a race track are often banked at an angle θ to allow the cars to achieve higher speeds around the turns. Assume friction is not present. Write an expression for the tan(θ) of a car going around the banked turn in terms of the car's speed v, the radius of the turn r, and g so that the car will not move up or down the incline of the turn. tan(θ) =arrow_forwardThe character Min Min from Arms was a DLC character added to Super Smash Bros. Min Min’s arms are large springs, with a spring constant of 8.53 ⋅ 10^3 N/m, which she uses to punch and fling away her opponents. Min Min pushes her spring arm against Steve, who is not moving, compressing it 1.20 m as shown in figure A. Steve has a mass of 81.6 kg. Assuming she uses only the spring to launch Steve, how fast is Steve moving when the spring is no longer compressed? As Steve goes flying away he goes over the edge of the level, as shown in figure C. What is the magnitude of Steve’s velocity when he is 2.00 m below where he started?arrow_forward
- Slinky dog whose middle section is a giant spring with a spring constant of 10.9 N/m. Woody, who has a mass of 0.412 kg, grabs onto the tail end of Slink and steps off the bed with no initial velocity and reaches the floor right as his velocity hits zero again. How high is the bed? What is Woody’s velocity halfway down? Enter just the magnitude of velocity.arrow_forwardNo chatgpt pls will upvotearrow_forwardA positive charge of 91 is located 5.11 m to the left of a negative charge 92. The charges have different magnitudes. On the line through the charges, the net electric field is zero at a spot 2.90 m to the right of the negative charge. On this line there are also two spots where the potential is zero. (a) How far to the left of the negative charge is one spot? (b) How far to the right of the negative charge is the other?arrow_forward
- A charge of -3.99 μC is fixed in place. From a horizontal distance of 0.0423 m, a particle of mass 7.31 x 103 kg and charge -9.76 µC is fired with an initial speed of 84.1 m/s directly toward the fixed charge. How far does the particle travel before its speed is zero?arrow_forwarda) What is the minimum tension in N that the cable must be able to support without breaking? Assume the cable is massless. T = b) If the cable can only support a tension of 10,000 N what is the highest mass the ball can have in kg? mm =arrow_forwardCurve Fitter CURVE FITTER Open Update Fit Save New Exclusion Rules Select Validation Data Polynomial Exponential Logarithmic Auto Fourier Fit Fit Duplicate Data Manual FILE DATA FIT TYPE FIT Harmonic Motion X us 0.45 mi ce 0.4 0.35 0.3 0.25 0.2 Residuals Plot Contour Plot Plot Prediction Bounds None VISUALIZATION Colormap Export PREFERENCES EXPORT Fit Options COA Fourier Equation Fit Plot x vs. t -Harmonic Motion a0+ a1*cos(x*w) + b1*sin(x*w) Number of terms Center and scale 1 ▸ Advanced Options Read about fit options Results Value Lower Upper 0.15 a0 0.1586 0.1551 0.1620 a1 0.0163 0.0115 0.0211 0.1 b1 0.0011 -0.0093 0.0115 W 1.0473 0.9880 1.1066 2 8 10 t 12 14 16 18 20 Goodness of Fit Value Table of Fits SSE 0.2671 Fit State Fit name Data Harmonic Motion x vs. t Fit type fourier1 R-square 0.13345 SSE DFE 0.26712 296 Adj R-sq 0.12467 RMSE 0.030041 # Coeff Valic R-square 0.1335 4 DFE 296.0000 Adj R-sq 0.1247 RMSE 0.0300arrow_forward
- What point on the spring or different masses should be the place to measure the displacement of the spring? For instance, should you measure to the bottom of the hanging masses?arrow_forwardLet's assume that the brightness of a field-emission electron gun is given by β = 4iB π² d²α² a) Assuming a gun brightness of 5x108 A/(cm²sr), if we want to have an electron beam with a semi-convergence angle of 5 milliradian and a probe current of 1 nA, What will be the effective source size? (5 points) b) For the same electron gun, plot the dependence of the probe current on the parameter (dpa) for α = 2, 5, and 10 milliradian, respectively. Hint: use nm as the unit for the electron probe size and display the three plots on the same graph. (10 points)arrow_forwardi need step by step clear answers with the free body diagram clearlyarrow_forward
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





