
Concept explainers
(a)
Interpretation: The percent by mass of M in
Concept introduction: Grignard reagent is an
To determine: The percent by mass of M in
(a)
Answer to Problem 173IP
Answer
The mass percent of M in
Explanation of Solution
Explanation
Given
Electronic configuration of
So,
In periodic table atomic number is found to be of the element Zinc.
The molar mass of Zinc is
The molar mass of compound
The molar mass of carbon is
The molar mass of hydrogen is
The molar mass of Zinc is
The molar mass of Bromine is
The molar mass of compound
Therefore the total molar mass of compound
The mass percent of M in
Substitute the value of the molar mass of M and total molar mass of compound, to calculate percent by mass of M in
Therefore, the mass percent of M in
Conclusion
The mass percent of M in
(b)
Interpretation: The percent by mass of M in
Concept introduction: Grignard reagent is an organometallic compound which is prepared by treating magnesium or metal atom with a strong acid. The Grignard reaction is defined as an organometallic reaction in which the vinyl, alkyl and aryl-metal halides were added to a carbonyl carbon atom and form an aldehyde or ketone.
To determine: The hybridization of the starred carbon atom in the given figure changed from reactants to products.
(b)
Answer to Problem 173IP
Answer
The hybridization of the starred carbon atom shown in Figure 1 is changed from
Explanation of Solution
Explanation
The given reaction is,
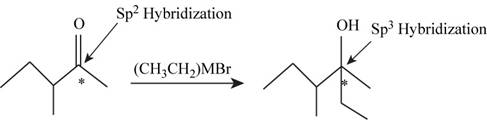
Figure 1
In the given reaction the reactant contains carbonyl group having double bonded oxygen atom hence, the hybridization of the starred carbon in the reactant is
Conclusion
The hybridization of the starred carbon atom shown in Figure 1 is changed from
(c)
Interpretation: The percent by mass of M in
Concept introduction: Grignard reagent is an organometallic compound which is prepared by treating magnesium or metal atom with a strong acid. The Grignard reaction is defined as an organometallic reaction in which the vinyl, alkyl and aryl-metal halides were added to a carbonyl carbon atom and form an aldehyde or ketone.
To determine: The systematic name of the product.
(c)
Answer to Problem 173IP
Answer
The systematic name of the product is
Explanation of Solution
Explanation
The given compound is,
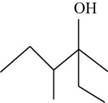
Figure 2
The longest carbon chain contains six carbon atoms numbered from the nearest
Conclusion
The systematic name of the product shown in Figure 2 is
Want to see more full solutions like this?
Chapter 22 Solutions
Bundle: Chemistry, Loose-leaf Version, 10th + Enhanced Webassign Printed Access Card For Chemistry, Multi-term Courses
- Q5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forwardCalculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forward
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




