
Concept explainers
A Boron and hydrogen form an extensive family of compounds, and the diagram below shows how they are related by reaction.
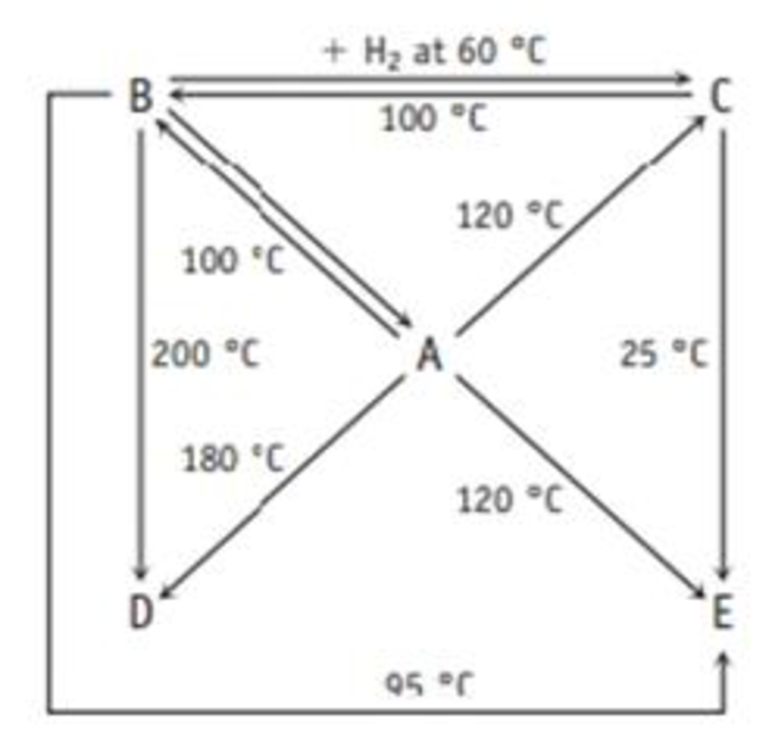
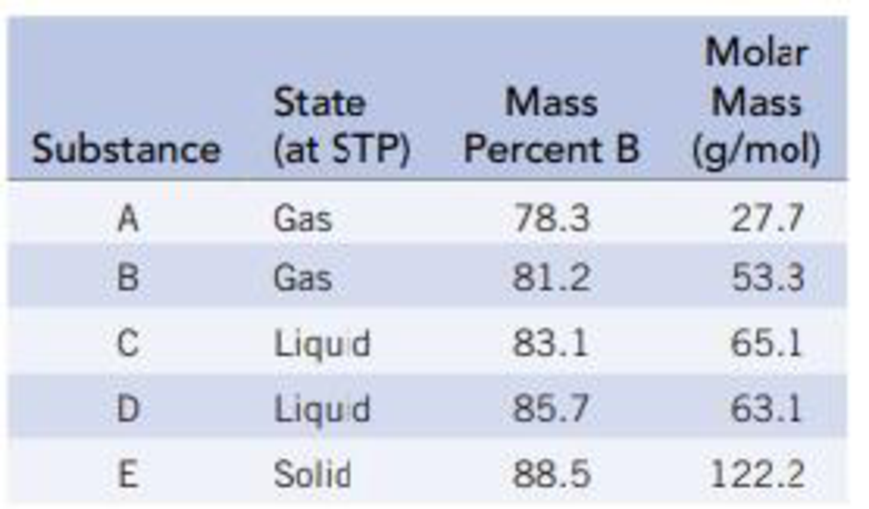
The following table gives the weight percent of boron in each of the compounds. Derive the empirical and molecular formulas of compounds A-E.
Interpretation: To determine the empirical and molecular formula of given compounds A-E.
Concept introduction:
The empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound.
A molecular formula shows the total number of atoms in a molecule but not their structural arrangement.
Answer to Problem 85GQ
The empirical formula of compound A is
The empirical formula of compound B is
The empirical formula of compound C is
The empirical formula of compound D is
The empirical formula of compound E is
Explanation of Solution
Boron and hydrogen form an extensive family of compounds. Substance A-E contains boron and hydrogen atoms.
The empirical and molecular formula of given compounds A-E is calculated below.
Given:
Substance A is a gaseous compound contains
The empirical formula of substance A is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound A is
The empirical formula molar mass of compound A is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance A by the number calculated above.
Thus, the molecular formula of substance A is
Substance B is a gaseous compound contains
The empirical formula of substance B is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound B is
The empirical formula molar mass of compound B is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance B by the number calculated above.
Thus, the molecular formula of substance B is
Substance C is a liquid compound contains
The empirical formula of substance C is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound C is
The empirical formula molar mass of compound A is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance C by the number calculated above.
Thus, the molecular formula of substance C is
Substance D is a liquid compound contains
The empirical formula of substance D is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound D is
The empirical formula molar mass of compound A is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance D by the number calculated above.
Thus, the molecular formula of substance D is
Substance E is a solid compound contains
The empirical formula of substance E is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound E is
The empirical formula molar mass of compound E is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance E by the number calculated above.
Thus, the molecular formula of substance E is
The empirical formula of compound A is
The empirical formula of compound B is
The empirical formula of compound C is
The empirical formula of compound D is
The empirical formula of compound E is
Want to see more full solutions like this?
Chapter 21 Solutions
CHEMISTRY+CHEM...HYBRID ED.(LL)>CUSTOM<
- Basic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





