
Interpretation:
How sodium perborate and sodium percarbonate work as bleaches must be explained.
Concept Introduction:
A bleaching agent is a substance that is used to decolorize other materials.
Colored materials have a group of atoms in their chemical structure that can absorb visible light of a particular color. These groups of atoms are known as chromophores. Chromophores absorb visible light of a particular color and reflect the complementary color.
Bleaching agents destroy the chromophores, by either oxidation or reduction.
Bleaching agents such as hypochlorite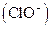 are oxidizing bleaches. Oxidizing bleaches work by breaking the
are oxidizing bleaches. Oxidizing bleaches work by breaking the
This is done by oxidizing the chromophore breaking the chemical bonds change the chromophore of a substance thereby decolorizing it.
Bleaches are either chlorine based, or oxygen based. Chlorine bleaches mainly contain hypochlorite and oxygen bleaches mainly contain perborates or percarbonates.
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Chemistry for Changing Times
- Chloroform, long used as an anesthetic and now considered carcinogenic, has a heat of vaporization of 31.4 kJ/mol. During vaporization, its entropy increases by 94.2 J/mol.K. Therefore, select the alternative that indicates the temperature, in degrees Celsius, at which chloroform begins to boil under a pressure of 1 atm. A) 28 B) 40 C) 52 D) 60 E) 72arrow_forwardIf we assume a system with an anodic overpotential, the variation of n as a function of current density: 1. at low fields is linear 2. at higher fields, it follows Tafel's law Obtain the range of current densities for which the overpotential has the same value when calculated for 1 and 2 cases (maximum relative difference of 5% compared to the behavior for higher fields). To which overpotential range does this correspond? Data: i = 1.5 mA cm², T = 300°C, B = 0.64, R = 8.314 J K1 mol-1 and F = 96485 C mol-1.arrow_forwardAnswer by equation pleasearrow_forward
- Some of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.arrow_forwardWhen talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?arrow_forwardUsing the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forward
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





