
Concept explainers
Interpretation:
Cosmetic containing bismuth oxychloride is a wise choice for skin health should be identified.
Concept introduction:
Minerals are naturally occurring chemical compound Titanium dioxide, 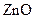 Mica, Iron oxides are the most commonly used minerals in cosmetics. Titanium oxide and
Mica, Iron oxides are the most commonly used minerals in cosmetics. Titanium oxide and 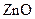 act as a sunblock so even application of mineral make up provide sun protection.
act as a sunblock so even application of mineral make up provide sun protection.
Silica a type of white clay used in ceramic and kaolin a mineral found in sandstone, clay, and granite used in cosmetic to absorb oil and moisture. Phenoxyethanol is a preservative used frequently in cosmetics, perfumes, and soaps.
Many leading brands include a preservative, parabens, mineral oils, chemicals dyes and fragrance in makeup, dermatologist recommend mineral make up based on the fact that these left out an ingredient and possible skin irritants.
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Chemistry for Changing Times
- In the decomposition reaction in solution B → C, only species C absorbs UV radiation, but neither B nor the solvent absorbs. If we call At the absorbance measured at any time, A0 the absorbance at the beginning of the reaction, and A∞ the absorbance at the end of the reaction, which of the expressions is valid? We assume that Beer's law is fulfilled.arrow_forward> You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: 1. ☑ CI 2. H3O+ O Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. Explanation Check ? DO 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardDon't use ai to answer I will report you answerarrow_forward
- Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence pointarrow_forwardWhat is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward
- 7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forwardIndicate the compound formula: dimethyl iodide (propyl) sulfonium.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





