
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (Chs 1-42) Plus Mastering Physics with Pearson eText -- Access Card Package (4th Edition)
4th Edition
ISBN: 9780133953145
Author: Randall D. Knight (Professor Emeritus)
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 21, Problem 38EAP
Prove that the work done in an adiabatic process i f is
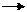 Ws= ( pfVf- piVi) / ( 1 - ? )
Ws= ( pfVf- piVi) / ( 1 - ? )
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Two blocks, A and B (with mass 45 kg and 120 kg, respectively), are connected by a string, as shown in the figure below. The pulley is frictionless and of negligible mass. The coefficient of kinetic friction between block A and the incline is μk = 0.26. Determine the change in the kinetic
energy of block A as it moves from to ①, a distance of 15 m up the incline (and block B drops downward a distance of 15 m) if the system starts from rest.
]
37°
A
©
B
A skateboarder with his board can be modeled as a particle of mass 80.0 kg, located at his center of mass. As shown in the figure below, the skateboarder starts from rest in a crouching position at one lip of a half-pipe (point). On his descent, the skateboarder moves without friction so
that his center of mass moves through one quarter of a circle of radius 6.20 m.
i
(a) Find his speed at the bottom of the half-pipe (point Ⓡ).
m/s
(b) Immediately after passing point Ⓑ, he stands up and raises his arms, lifting his center of mass and essentially "pumping" energy into the system. Next, the skateboarder glides upward with his center of mass moving in a quarter circle of radius 5.71 m, reaching point D. As he
passes through point ①, the speed of the skateboarder is 5.37 m/s. How much chemical potential energy in the body of the skateboarder was converted to mechanical energy when he stood up at point Ⓑ?
]
(c) How high above point ① does he rise?
m
A 31.0-kg child on a 3.00-m-long swing is released from rest when the ropes of the swing make an angle of 29.0° with the vertical.
(a) Neglecting friction, find the child's speed at the lowest position.
m/s
(b) If the actual speed of the child at the lowest position is 2.40 m/s, what is the mechanical energy lost due to friction?
]
Chapter 21 Solutions
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (Chs 1-42) Plus Mastering Physics with Pearson eText -- Access Card Package (4th Edition)
Ch. 21 - Prob. 1CQCh. 21 - Rank in order, from largest to smallest, the...Ch. 21 - Prob. 3CQCh. 21 - FIGURE Q21.4 shows the pV diagram of a heat...Ch. 21 - Rank in order, from largest to smallest, the...Ch. 21 - FIGURE Q21.6 shows the thermodynamic cycles of two...Ch. 21 - A heat engine satisfies Wout= Qnet. Why is there...Ch. 21 - Prob. 8CQCh. 21 - Prob. 9CQCh. 21 - Prob. 10CQ
Ch. 21 - Prob. 11CQCh. 21 - Prob. 1EAPCh. 21 - Prob. 2EAPCh. 21 - Prob. 3EAPCh. 21 - Prob. 4EAPCh. 21 - Prob. 5EAPCh. 21 - Prob. 6EAPCh. 21 - The power output of a car engine running at 2400...Ch. 21 - Prob. 8EAPCh. 21 - Prob. 9EAPCh. 21 - Prob. 10EAPCh. 21 - Prob. 11EAPCh. 21 - Prob. 12EAPCh. 21 - Prob. 13EAPCh. 21 - Prob. 14EAPCh. 21 - Prob. 15EAPCh. 21 - Prob. 16EAPCh. 21 - A heat engine uses a diatomic gas in a Brayton...Ch. 21 - At what pressure ratio does a Brayton cycle using...Ch. 21 - Prob. 19EAPCh. 21 - Prob. 20EAPCh. 21 - Prob. 21EAPCh. 21 - Prob. 22EAPCh. 21 - Prob. 23EAPCh. 21 - Prob. 24EAPCh. 21 - Prob. 25EAPCh. 21 - Prob. 26EAPCh. 21 - Prob. 27EAPCh. 21 - A Carnot engine whose hot-reservoir temperature is...Ch. 21 - Prob. 29EAPCh. 21 - A heat engine operating between energy reservoirs...Ch. 21 - Prob. 31EAPCh. 21 - A Carnot refrigerator operating between —20°C and...Ch. 21 - The coefficient of performance of a refrigerator...Ch. 21 - A Carnot heat engine with thermal efficiency 1/3...Ch. 21 - Prob. 35EAPCh. 21 - Prob. 36EAPCh. 21 - A heat engine with 50% of the Carnot efficiency...Ch. 21 - Prove that the work done in an adiabatic process i...Ch. 21 - Prob. 39EAPCh. 21 - Prob. 40EAPCh. 21 - An ideal refrigerator utilizes a Carnot cycle...Ch. 21 - Prob. 42EAPCh. 21 - There has long been an interest in using the vast...Ch. 21 - A Carnot heat engine operates between reservoirs...Ch. 21 - A Carnot engine operates between temperatures of...Ch. 21 - Prob. 46EAPCh. 21 - A Carnot heat engine and an ordinary refrigerator...Ch. 21 - 48. A heat engine running backward is called a...Ch. 21 - 49. A car's internal combustion engine can be...Ch. 21 - Prob. 50EAPCh. 21 - Prob. 51EAPCh. 21 - Prob. 52EAPCh. 21 - Prob. 53EAPCh. 21 - Prob. 54EAPCh. 21 - Prob. 55EAPCh. 21 - Prob. 56EAPCh. 21 - Prob. 57EAPCh. 21 - A heat engine using a monatomic gas follows the...Ch. 21 - Prob. 59EAPCh. 21 - Prob. 60EAPCh. 21 - Prob. 61EAPCh. 21 - Prob. 62EAPCh. 21 - Prob. 63EAPCh. 21 - Prob. 64EAPCh. 21 - Prob. 65EAPCh. 21 - Prob. 66EAPCh. 21 - Prob. 67EAPCh. 21 - Prob. 68EAPCh. 21 - Prob. 69EAPCh. 21 - Prob. 70EAPCh. 21 - A refrigerator using helium gas operates on the...Ch. 21 - Prob. 72EAPCh. 21 - The gasoline engine in your car can be modeled as...Ch. 21 - Prob. 74EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A force acting on a particle moving in the xy plane is given by F = (2yî + x²), where F is in newtons and x and y are in meters. The particle moves from the origin to a final position having coordinates x = 5.60 m and y = 5.60 m, as shown in the figure below. y (m) B (x, y) x (m) (a) Calculate the work done by F on the particle as it moves along the purple path (0 Ⓐ©). ] (b) Calculate the work done by ♬ on the particle as it moves along the red path (0 BC). J (c) Is F conservative or nonconservative? ○ conservative nonconservativearrow_forwardA 3.5-kg block is pushed 2.9 m up a vertical wall with constant speed by a constant force of magnitude F applied at an angle of 0 = 30° with the horizontal, as shown in the figure below. If the coefficient of kinetic friction between block and wall is 0.30, determine the following. (a) the work done by F J (b) the work done by the force of gravity ] (c) the work done by the normal force between block and wall J (d) By how much does the gravitational potential energy increase during the block's motion? ]arrow_forwardPhysics different from a sea breeze from a land breezearrow_forward
- File Preview Design a capacitor for a special purpose. After graduating from medical school you and a friend take a three hour cruise to celebrate and end up stranded on an island. While looking for food, a spider falls on your friend giving them a heart attack. Recalling your physics, you realize you can build a make-shift defibrillator by constructing a capacitor from materials on the boat and charging it using the boat's battery. You know that the capacitor must hold 100 J of energy and be at 1000 V (fortunately this is an electric boat which has batteries that are 1000 V) to work. You decide to construct the capacitor by tightly sandwiching a single layer of Saran wrap between sheets of aluminum foil. You read the Saran wrap box and fortunately they tell you that it has a thickness 0.01 mm and dielectric constant of 2.3. The Saran wrap and foil are 40 cm wide and very long. How long is the final capacitor you build that saves your friend?arrow_forwardHow do I plot the force F in Matlba (of gravity pulling on the masses) versus spring displacement, and fit the data with a linear function to find the value for the spring constant. To get a linear fit, use polynomial order 1. Report the value of 'k' from the fit. What code is used?arrow_forwardOk im confused on this portion of the questions being asked. the first snip is the solution you gave which is correct. BUt now it is asking for this and im confused. The magnitude of the force F_11 is __________LB. The direction of the force F_11 is __________LB.arrow_forward
- Solve and answer the problem correctly and be sure to check your work. Thank you!!arrow_forwardThe spring in the figure has a spring constant of 1300 N/m. It is compressed 17.0 cm, then launches a 200 g block. The horizontal surface is frictionless, but the block’s coefficient of kinetic friction on the incline is 0.200. What distance d does the block sail through the air?arrow_forwardSolve and answer the problem correctly and be sure to check your work. Thank you!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning
Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Classical Dynamics of Particles and Systems
Physics
ISBN:9780534408961
Author:Stephen T. Thornton, Jerry B. Marion
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY