
ORGANIC CHEMISTRY-ACCESS
6th Edition
ISBN: 9781260475586
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 32P
Draw enol tautomer(s) for each compound.
a. 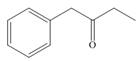 b.
b. 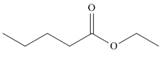 c.
c. 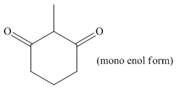
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the Lewis structure of C2H4O
a)
5. Circle all acidic (and anticoplanar to the Leaving group) protons in the
following molecules, Solve these elimination reactions, and identify the
major and minor products where appropriate: 20 points
+
NaOCH3
Br
(2 product
None
Chapter 21 Solutions
ORGANIC CHEMISTRY-ACCESS
Ch. 21.2 - Problem 23.1 Draw the enol or keto tautomer(s) of...Ch. 21.2 - Problem 23.3 When phenylacetaldehyde is dissolved...Ch. 21.3 - Prob. 5PCh. 21.3 - Problem 23.5 Which bonds in the following...Ch. 21.3 - Prob. 7PCh. 21.3 - Prob. 8PCh. 21.3 - Prob. 9PCh. 21.4 - Prob. 10PCh. 21.5 - Prob. 11PCh. 21.7 - Problem 23.11 Draw the products of each...
Ch. 21.7 - Problem 23.12 Draw the products of each reaction....Ch. 21.7 - Prob. 14PCh. 21.7 - Prob. 15PCh. 21.8 - Prob. 16PCh. 21.8 - Prob. 17PCh. 21.8 - Prob. 18PCh. 21.8 - Problem 23.18 How can pentan-2-one be converted...Ch. 21.8 - Problem 23.19 Identify A, B, and C, intermediates...Ch. 21.9 - Problem 23.20 Which of the following compounds...Ch. 21.9 - Problem 23.21 Draw the products of each...Ch. 21.9 - Prob. 23PCh. 21.9 - Prob. 24PCh. 21.9 - Prob. 25PCh. 21.10 - Prob. 26PCh. 21.10 - Prob. 27PCh. 21.10 - Prob. 28PCh. 21.10 - Prob. 29PCh. 21 - 23.29 Draw enol tautomer(s) for each compound....Ch. 21 - 22.30 The cis ketone A is isomerized to a trans...Ch. 21 - 23.31 Draw enol tautomer(s) for each compound.
...Ch. 21 - Prob. 33PCh. 21 - Prob. 34PCh. 21 - 23.35 Rank the labeled protons in each compound in...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - 23.38 Acyclovir is an effective antiviral agent...Ch. 21 - 23.39 Explain why forms two different alkylation...Ch. 21 - Prob. 40PCh. 21 - 23.42 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 42PCh. 21 - Prob. 43PCh. 21 - 23.45 Devise a synthesis of valproic acid , a...Ch. 21 - Prob. 57PCh. 21 - 23.57 Draw a stepwise mechanism showing how two...Ch. 21 - 23.58 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 65PCh. 21 - 23.66 Synthesize (Z)-hept-5-en-2-one from ethyl...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Dr. Mendel asked his BIOL 260 class what their height was and what their parent's heights were. He plotted that data in the graph below to determine if height was a heritable trait. A. Is height a heritable trait? If yes, what is the heritability value? (2 pts) B. If the phenotypic variation is 30, what is the variation due to additive alleles? (2 pts) Offspring Height (Inches) 75 67.5 60 52.5 y = 0.9264x + 4.8519 55 60 65 MidParent Height (Inches) 70 75 12pt v V Paragraph B IUA > AT2 v Varrow_forwardExperiment: Each team will be provided with 5g of a mixture of acetanilide and salicylic acid. You will divide it into three 1.5 g portions in separate 125 mL Erlenmeyer flasks savıng some for melting point analysis. Dissolve the mixture in each flask in ~60mL of DI water by heating to boiling on a hotplate. Take the flasks off the hotplate once you have a clear solution and let them stand on the bench top for 5 mins and then allow them to cool as described below. Sample A-Let the first sample cool slowly to room temperature by letting it stand on your lab bench, with occasional stirring to promote crystallization. Sample B-Cool the second sample 1n a tap-water bath to 10-15 °C Sample C-Cool the third sample in an ice-bath to 0-2 °C Results: weight after recrystalization and melting point temp. A=0.624g,102-115° B=0.765g, 80-105° C=1.135g, 77-108 What is the percent yield of A,B, and C.arrow_forwardRel. Intensity Q 1. Which one of the following is true of the compound whose mass spectrum is shown here? Explain how you decided. 100 a) It contains chlorine. b) It contains bromine. c) It contains neither chlorine nor bromine. 80- 60- 40- 20- 0.0 0.0 TT 40 80 120 160 m/z 2. Using the Table of IR Absorptions how could you distinguish between these two compounds in the IR? What absorbance would one compound have that the other compound does not? HO CIarrow_forward
- Illustrate reaction mechanisms of alkenes with water in the presence of H2SO4, detailing each step of the process. Please show steps of processing. Please do both, I will thumb up for sure #1 #3arrow_forwardDraw the following molecule: (Z)-1-chloro-1-butenearrow_forwardIdentify the molecule as having a(n) E, Z, cis, or trans configuration. CH3 H₁₂C ○ E ○ z ○ cis transarrow_forward
- Identify the molecule as having a(n) E, Z, cis, or trans configuration. H₂C- CH3 О Е ○ cis ○ transarrow_forwardThe decomposition of dinitrogen pentoxide according to the equation: 50°C 2 N2O5(g) 4 NO2(g) + O2(g) follows first-order kinetics with a rate constant of 0.0065 s-1. If the initial concentration of N2O5 is 0.275 M, determine: the final concentration of N2O5 after 180 seconds. ...arrow_forwardDon't used hand raitingarrow_forward
- CS2(g) →CS(g) + S(g) The rate law is Rate = k[CS2] where k = 1.6 × 10−6 s−¹. S What is the concentration of CS2 after 5 hours if the initial concentration is 0.25 M?arrow_forwardCS2(g) → CS(g) + S(g) The rate law is Rate = k [CS2] where k = 1.6 × 10-6 s−1. S Calculate the half-life.arrow_forwardThe following is a first order reaction where the rate constant, k, is 6.29 x 10-3 min-*** What is the half-life? C2H4 C2H2 + H2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY